Transcatheter Aortic Valve Replacement (TAVR): Development of the Polymer Valve
Designing polymer valves for extended durability and improved hemodynamics
Transcatheter aortic valve replacement (TAVR) has recently become a life-saving minimally-invasive alternative to patients who cannot tolerate open-heart surgical valve replacement. FDA-approved TAVR devices such as the Edwards Lifesciences Sapien and Medtronic CoreValve consist of a metal stent and bioprosthetic xenograft leaflets. However, associated complications and poor durability impede current TAVR technology from expanding into younger, lower-risk, operable patients.
Polymer valves present a clear alternative and advance over tissue valves, offering a large degree of design flexibility, material properties, low production costs, and higher tolerance for damages that the valve may incur during TAVR deployment. Ideally, polymer valves can offer the extended durability of mechanical valves together with the hemodynamic performance of bioprosthetic valves. Efforts by several groups have yet to successfully combine the properties needed for a successful prosthetic heart valve - biostability, low thrombogenicity, calcification resistance, hydrodynamics, durability, and manufacturing consistency.
We are developing a TAVR valve using a novel polymer technology and design optimization concept.
The Polymer
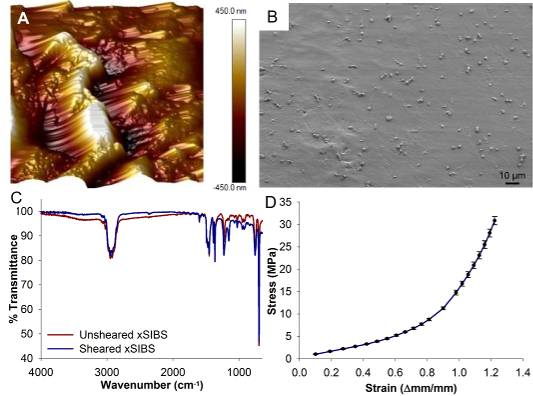
xSIBS - a thermoset, cross-linked, variation of SIBS designed for use in heart valves by Innovia LLC (Miami, Florida), with mechanical properties and hemocompatibility evaluated by our group. It maintains the low thrombogenic potential of SIBS, while having drastically improved mechanical properties, as required for prosthetic heart valve applications.
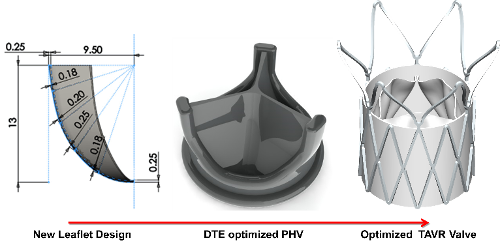
Valve Design
- Optimized through analysis with device thrombogenicity emulation (DTE) methodology for achieving a reduced clotting risk.
- Variable radial cross-sectional thickness of the leaflet to minimize stresses developed in the leaflets under a diastolic pressure load and during leaflet flexion.
- Design of the compression mold such that the manufactured leaflets experience minimal stresses during their excursion to fully open and closed positions for achieving better hemodynamic performance combined with extended durability.
PolyV-B: Dedicated TAVR Valve for BAV Patients
- Bicuspid aortic valve (BAV), the most common congenital heart malformation. BAV often leads to early onset of calcific aortic stenosis (AS). Despite the increasing use of TAVR in BAV, pathological BAV anatomy often leads to complications stemming from mismatched anatomical features. To mitigate these complications, a novel eccentric polymeric TAVR valve incorporating asymmetrical leaflets was designed specifically for BAV anatomies.
- Computational modeling was used to optimize its asymmetric leaflets for lower functional stresses and improved hemodynamic performance. Deployment and flow were simulated in patient-specific BAV models and compared to a current commercial TAVR valve to assess deployment and flow parameters.
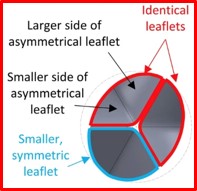
- The novel eccentric BAV-dedicated valve demonstrated significant improvements in peak systolic orifice area, along with lower jet velocity and wall shear stress. This feasibility study demonstrates the clinical potential of the first known BAV-dedicated TAVR design, which will foster advancement of patient-dedicated valvular devices.
- The present work is a ‘proof of concept’ feasibility study that will serve for prototype manufacturing and testing. A dedicated stent design is currently being developed to not only fit the asymmetric leaflet design, but also provide preferential force distirbution along the major and minor axes of the elliptical orifice the device will be deployed in. This is because the novel valve’s asymmetric leaflets need to be oriented such that the long axis of the leaflets aligns with the long axis of the BAV’s elliptical orifice. A skirt design is also being developed for this specialized design.
