Multiscale Modeling of Thrombosis
Modeling dynamic platelet response to flow conditions
Elucidation of the complex interactions between living tissues and mechanical stimuli, as represented by the vexing problem of shear-mediated platelet activation and resultant thrombosis, can be realized by innovative multiscale modeling (MSM) approaches.
The coupling of the disparate spatial-temporal scales between the macroscopic transport and the molecular level events represents a major modeling and computational challenge. Continuum approaches can be effectively utilized only down to the micron level and are limited in their ability to cover the smaller molecular mechanisms such as filopodia formation during platelet activation. Utilizing molecular dynamics (MD) to cover the multiscales involved is computationally prohibitive.
We have developed an integrated MSM methodology that addresses the mechano-biological “linkages,” and cover the vast range of spatiotemporal scales involved:
- Dissipative Particle Dynamics (DPD) model of viscous blood flow, interfaced with a bottom Coarse Grained Molecular Dynamics (CGMD) model of platelets. This integrated model simulates their activation process via mechanotransduction pathways
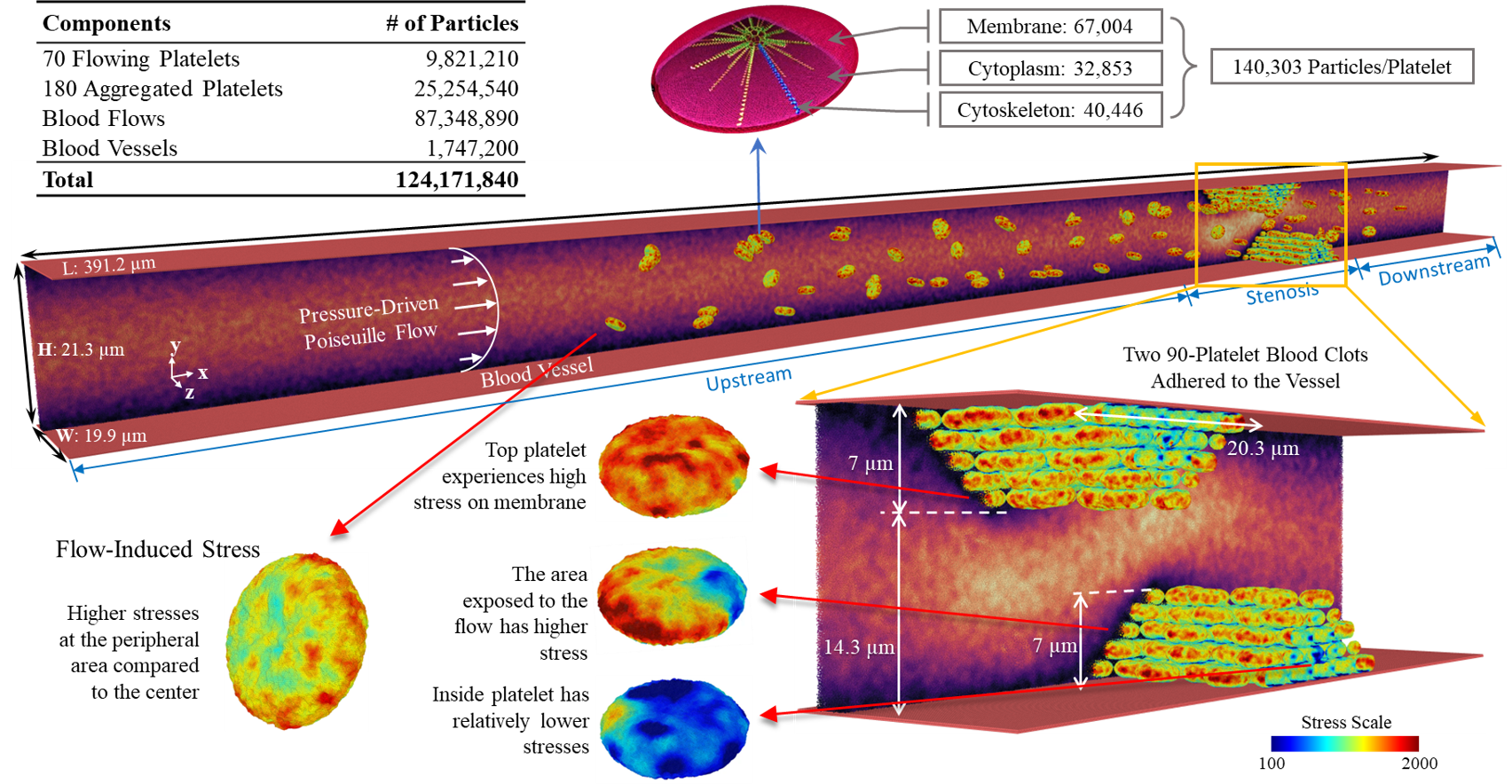
- Platelets are composed of internal structural and functional components (bilayer membrane, microtubules, cytoplasm, and cytoskeleton)
- Fully interactive interface between the top and bottom models through an hybrid force field interface and a multiple time stepping (MTS) scheme for handling the temporal disparity
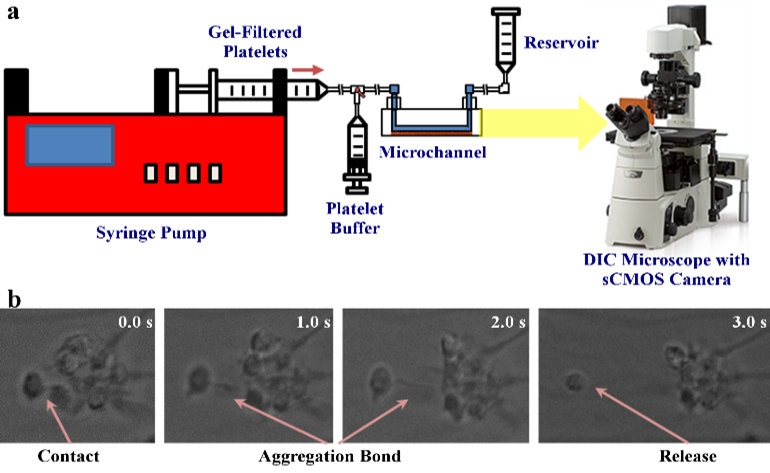
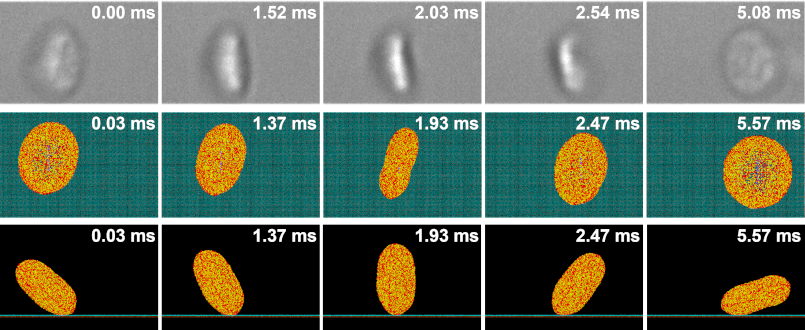
- Validation of computational models with in vitro experiments of platelet shape change and motion, performed using the hemodynamic shearing device (HSD) and microchannels. Images are obtained using scanning electron microscopy (SEM) and DIC microscopy with a high framerate sCMOS camera
Multiscale Shear-Mediated Platelet Aggregation
One of the earlier stages in clot formation is platelet-platelet aggregation, which is primarily mediated by the interaction of integrin αIIbβ3 receptors on the platelet membrane via fibrinogen. The platelet membrane in our model includes 67,004 integrin αIIbβ3 receptors represented by particles and distributed uniformly on the membrane. The aggregation force field between the receptor particles of different platelets is modeled via a combination of the Morse potential and Hooke force and simulated under flow conditions.
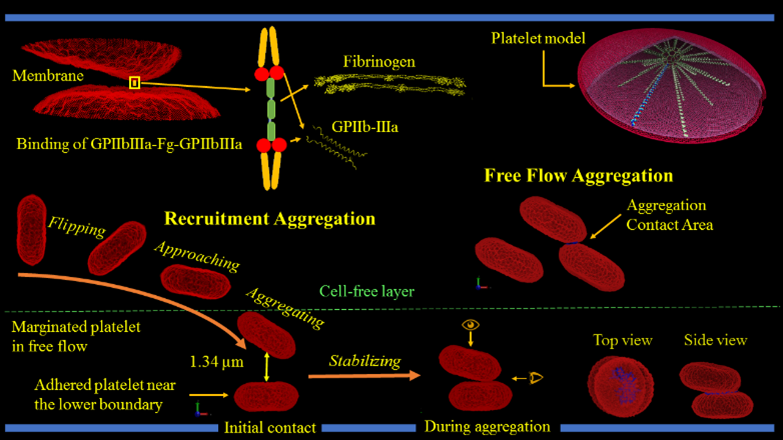
We validate our numerical method by correlating numerical simulations and model predictions with in vitro results. For aggregation experiments, we mix purified platelets with isolated red blood cells, perfuse through microchannels precoated with von Willebrand factor using a syringe pump, and allow marginated platelets to adhere to the channel walls. We then perfuse purified platelets mixed with fibrinogen over adhered platelets to initiate aggregation interactions. Aggregation events are observed at 100× magnification under a DIC microscope at up to 2000 frames per second.
Multiscale Shear-Mediated Platelet Adhesion
Platelet adhesion to blood vessel walls in shear flow is essential to initiating the blood coagulation cascade and prompting clot formation in vascular disease processes and prosthetic cardiovascular devices. In the adhesion process, platelets undergo complex and rapid receptor-ligand binding such as bond association and breakage.
We adapt our deformable CGMD multiscale platelet model to describe flipping over von Willebrand Factor (vWF) in a channel with DPD particles at several shear stresses. Approximately a quarter of the platelet’s bilayer membrane particles are designated as GPIbα receptors, which are redistributed on the membrane according to local membrane curvature. The vWF , seeded on a square-lattice 3-dimensional deformable plane, binds to a GPIbα receptor and initiates adhesion. The force field between the GPIbα and the vWF ligand is described by a parameterized hybrid force field combing Morse and Hooke potentials.
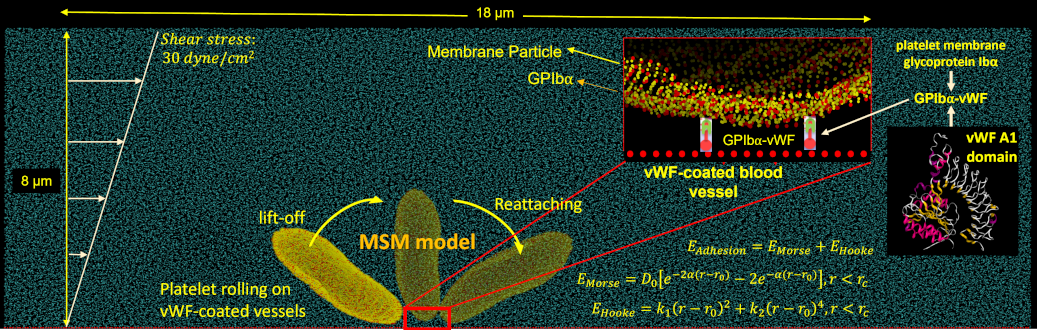
As in our multiscale aggregation simulations, we validate our adhesion models in vitro. Purified platelets are perfused at various shear stresses through microchannels pre-coated with vWF using a syringe pump. Adhesion events, defined as platelets flipping or sliding on the vWF-coated surface, are observed at 100× magnification on a DIC microscope (Ti-E, Nikon) up to 2000 fps (Zyla, Andor Technology Ltd).
We designed and trained a semi-unsupervised learning system (SULS), consisting of multiple convolutional neural networks, to extract and classify platelet morphological features from captured images. In vitro platelet adhesion images segmented by the SULS yields a time series of 2D geometrical data points, which are then input into a mathematical model to calculate the previously described geometrical parameters, as well as the platelets’ rolling kinematics and allows prediction of bond dynamics via our multiscale modeling approach.
Multi-platelet Clot Formation
We simulate a large-scale multiscale system of a shear flow-mediated forming thrombus with up to 250 platelets (70 flowing and 180 aggregating), consisting of 124M particles. This set-up is integrated with our Artificial Intelligence-Multiscale Modeling (AI-MSM) framework. The blood vessel walls are modeled with a no-slip boundary conditions with a pressure-driven Poiseuille blood flow between the top and bottom plates. Two blood adhered clots, each with 90 aggregated platelets, formed a stenosis, with aggregation between receptors of the platelets is mediated by the membrane αIIbβ3 receptors and fibrinogen, governed by a combined Morse potential and Hooke force field.
Our AI-MTS algorithm splits the system into two subsystems based on the spatial scales of the underlying components: the macroscopic fluid and the microscopic platelet. By collecting multiple time series properties describing platelet dynamics, we predict the adaptive timestep sizes and number of jumps via a deep learning framework consisting of (a) 2-stage denoising and (b) RNN-based autoencoders to extract latent features.