Past Projects
Thrombogenic Potential of Prosthetic Heart Valves
Patients with severe valvular diseases require heart valve replacement as a necessary treatment. Approximately 2 million individuals have received prosthetic heart valves (PHVs), with 120,000 valves implanted annually in the United States. About 60% of PHVs are categorized as mechanical heart valves (MHVs), with the remainder described as bioprosthetic or polymer valves. Each valve type has its advantages and disadvantages, with primary focus being placed on the risk of thromboembolism due to platelet activation induced by non-physiologic flow patterns, typically blamed on valve leaflet design. Polymer valves have been touted as a solution to this issue, owing to the design of leaflets that mimic geometry found in natural valves, and newer products utilize materials that show high biocompatibility and reduced long-term degradation. Our objective is to quantify the thrombogenic potential of 3 types of PHVs via platelet activation, and to identify which valve is least likely to cause long-term thromboembolic complications. The three valves utilized in our study are the St. Jude Medical bileaflet MHV, a Carpentier-Edwards bioprosthetic valve, and a trileaflet polymer valve, provided by Dr. Richard Schoephoerster. The valves are mounted left ventricular assist device (LVAD), kindly provided by Dr. Klaus Affeld, filled with a platelet mixture at physiological concentration, and connected to a Harvard Apparatus pulsatile blood pump. We conduct 30-minute experiments, with platelets sampled at regular intervals for the platelet activation state (PAS) assay, which allows quantification of platelet activation, and therefore thrombogenic potential of each valve. Previous studies examined platelet activation in 3 PHVs of prior designs, as well as the thrombogenic potential of bileaflet and monoleaflet MHVs.
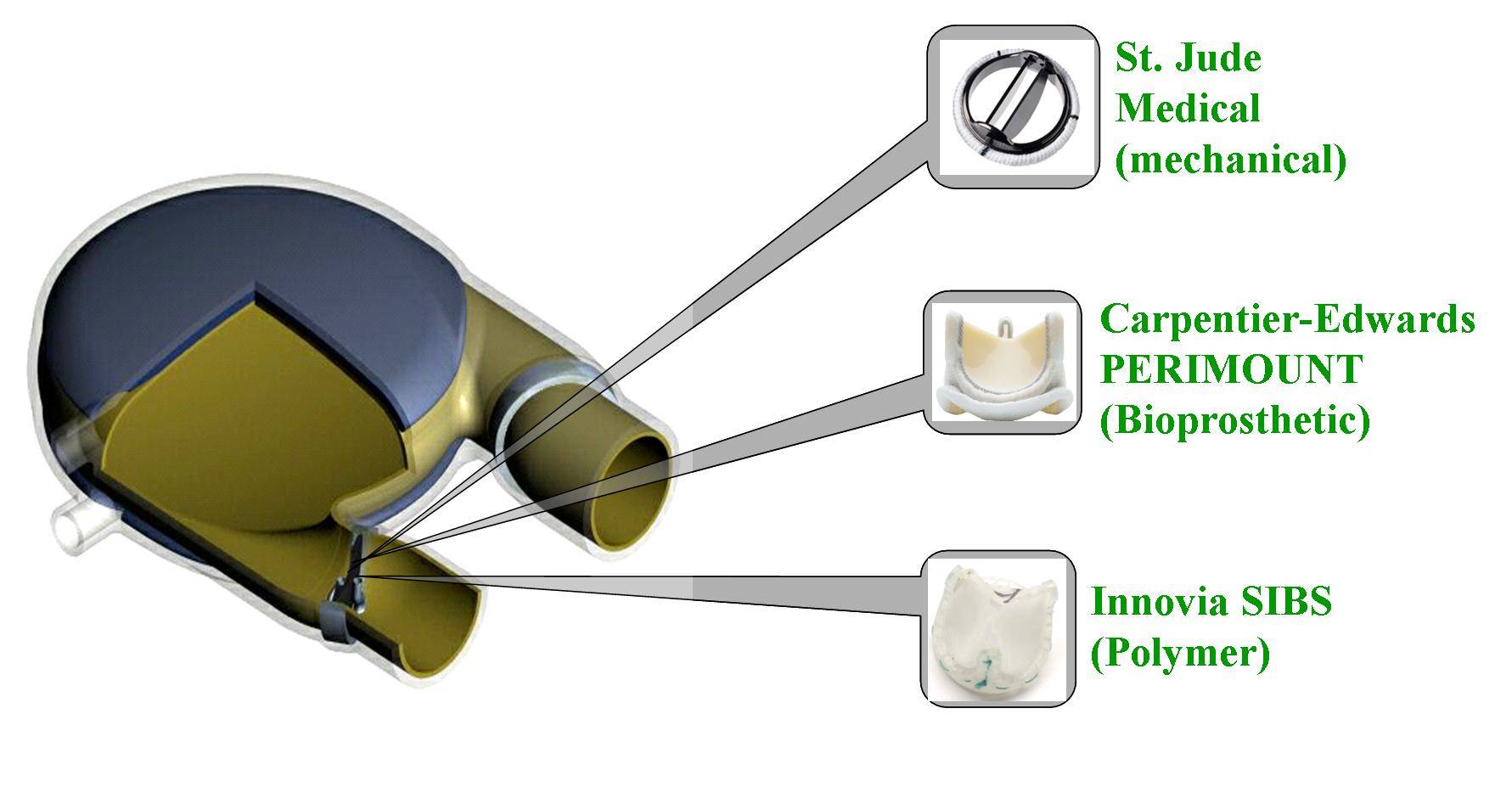
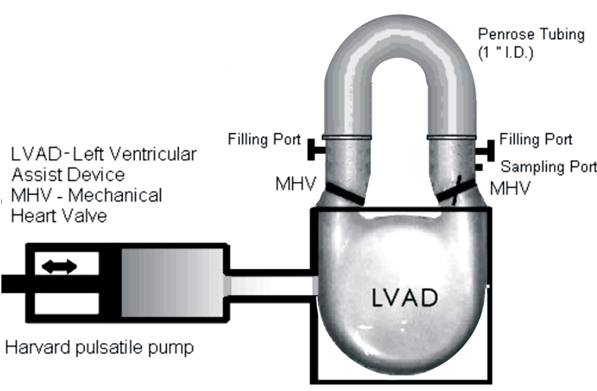
Modeling the Thrombogenic Potential of Mechanical Heart Valves
Mechanical heart valves (MHVs) make up approxmiately % of prosthetic heart valve (PHV) implants in the United States. Due to the high risk of cardioembolic stroke, patents receiving MHV implants are administered mandatory anticoagulation medication. However, this increases the risk of hemorrhage and does not eliminate risk of stroke. This study is based on the blood flow conditions present in MHVs. Computational fluid dynamics (CFD) tools are used to study flow through an MHV from St. Jude Medical (SJM) after implantation in physiologic 3D geometry. In this study, blood was taken to be a non-Newtonian two-phase fluid experiencing intermittent turbulence. The simulation is conducted using the Unsteady Reynolds Averaged Navier-Stokes (URANS) approach that employs the Wilcox k-ω turbulent model, which is able to account for intermittency of the turbulence. The results of the flow simulation are used to develop a new platelet damage model incorporating damage history (senescence and cumulative damage of repeated passages). This model is used to determine platelet activation resulting from combined stress and exposure time.
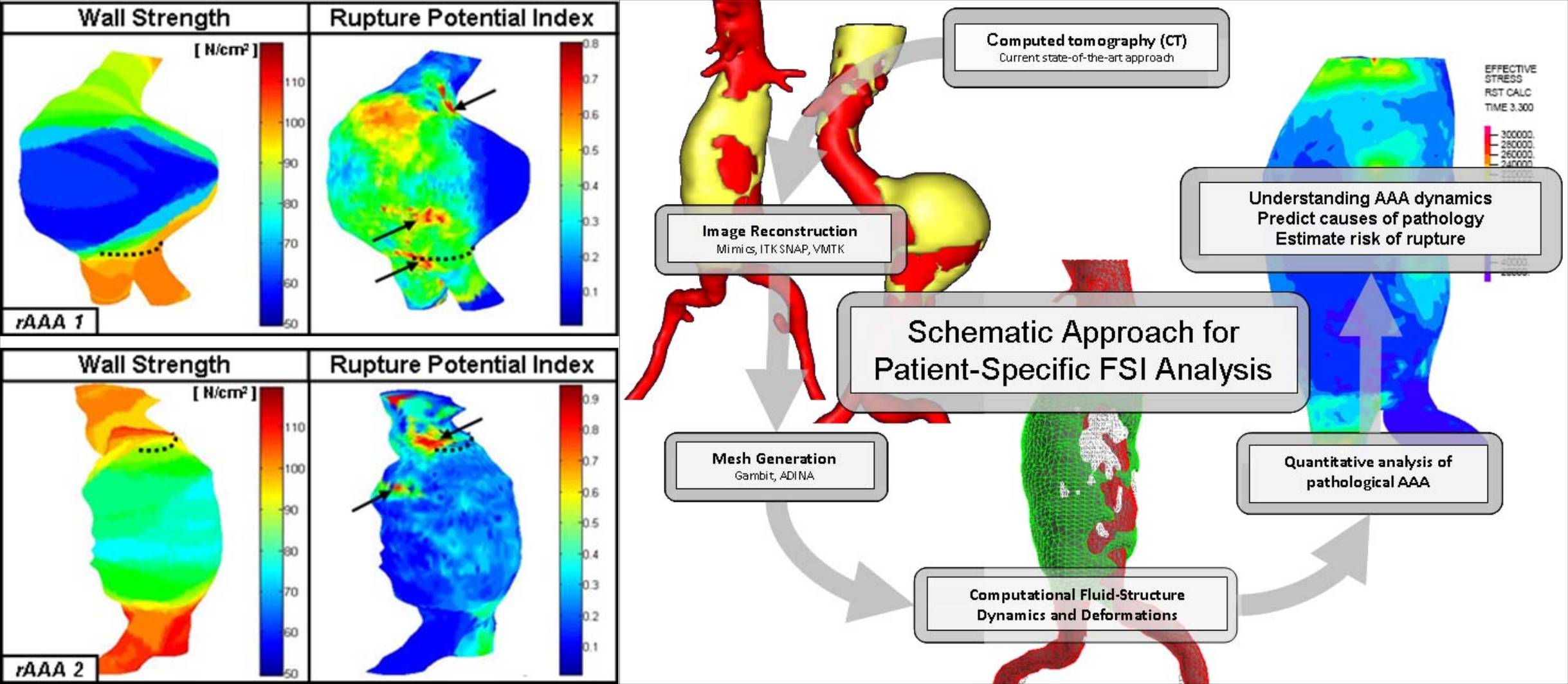
Abdominal Aortic Aneurysm (AAA) from Patient-Generated Images
Abdominal aortic aneurysm (AAA) is an irreversible dilation, i.e., ballooning, of an artery due to gradual wall weakening. It represents a common vascular pathology with possibly fatal implications in which the aneurysm expands and may eventually rupture. In the current clinical management of AAA patients, the maximum transverse dimension of the aneurysm (5.5 cm diameter) is often used as the primary indicator of rupture potential. Repair is warranted when the risk of rupture exceeds that of the repair as to justify an elective surgical resection. There is a need to more accurately predict the risk of rupture based on patient specific parameters, because aneurysms smaller than 5.5 cm rupture and larger ones can remain intact. Consequently, patient specific AAA FSI simulations are carried out, with anisotropic wall materials. The use of an anisotropic material model used closely models experimental results for the stress strain relationship. Results show peak wall stresses are dependent on the shape of the AAA geometry and the region of highest stress corresponds to expected failure location.
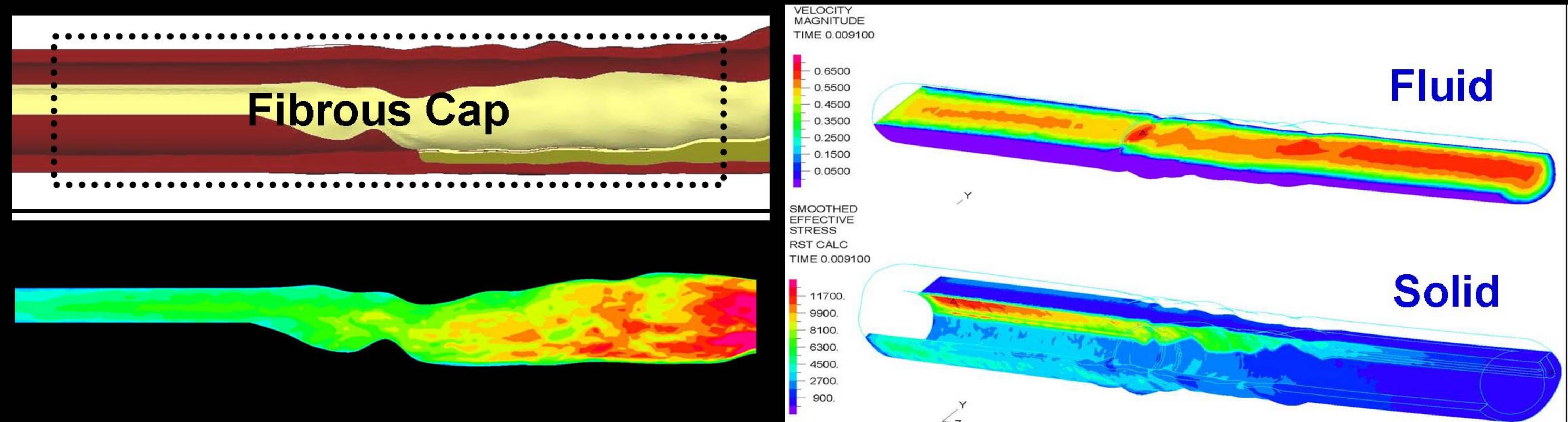
Fluid Structure Interaction (FSI) Modeling of Vulnerable Plaque
Coronary artery disease (CAD) is the single largest killer of American males and females, and total costs directly and indirectly attributed to the disease are estimated to be $142.1 billion in 2005. One important manifestation of this disease, the vulnerable plaque, is characterized by a thin fibrous cap covering a lipid core, and is responsible for sudden cardiac death in patients with no visible symptoms. This has been imaged extensively by both invasive and non-invasive methods for characterizing atherosclerotic lesions and the development of diagnostic and drug treatment analysis techniques. However, there is lack of in vivo, specifically imaging, techniques to accurately predict potential sites of vulnerable plaque rupture. Therefore, investigators have to resort to examination of histological samples or Finite Element (FE) techniques, based on imaging of the atherosclerotic lesion. Intravascular Ultrasound (IVUS) images of atherosclerotic plaques, provided by the Division of Cardiovascular Medicine at Stony Brook University, are processed by the Vulcano virtual histology software, which classifies various regions of the plaque according to tissue type. We use the MATLAB and Gambit software packages to define and recreate a 3D model of the plaque from these images. The ADINA fluid structure interaction (FSI) package is then utilized to simulate flow through the coronary artery of which the vulnerable plaque is a part. Our long term objective is to examine the effect of microcalcification and fibrous cap thickness on the risk of vulnerable plaque rupture.
Smoking and Enhanced Platelet Activation
Cigarette smoke has been recognized as a primary risk factor for the onset of certain cardiovascular diseases and their progression. Specifically, the risks of acquiring coronary heart disease and peripheral vascular disease are increased greatly due to exposure to cigarette smoke. It has also been demonstrated that cigarette smoke increases platelet thrombus formation in patients with coronary artery disease. Platelet function is greatly enhanced in smokers, leading to excessive platelet-dependent thrombin generation, and thereby placing them at increased thrombotic risk. The risks associated with cigarette smoke are not confined to smokers alone, as nonsmokers who are exposed to the same by means of environmental tobacco smoke (ETS), or secondhand smoke (SHS) (smoke obtained mainly from the smoldering end of the cigarette, a.k.a Sidestream) also show a high thrombogenic potential.
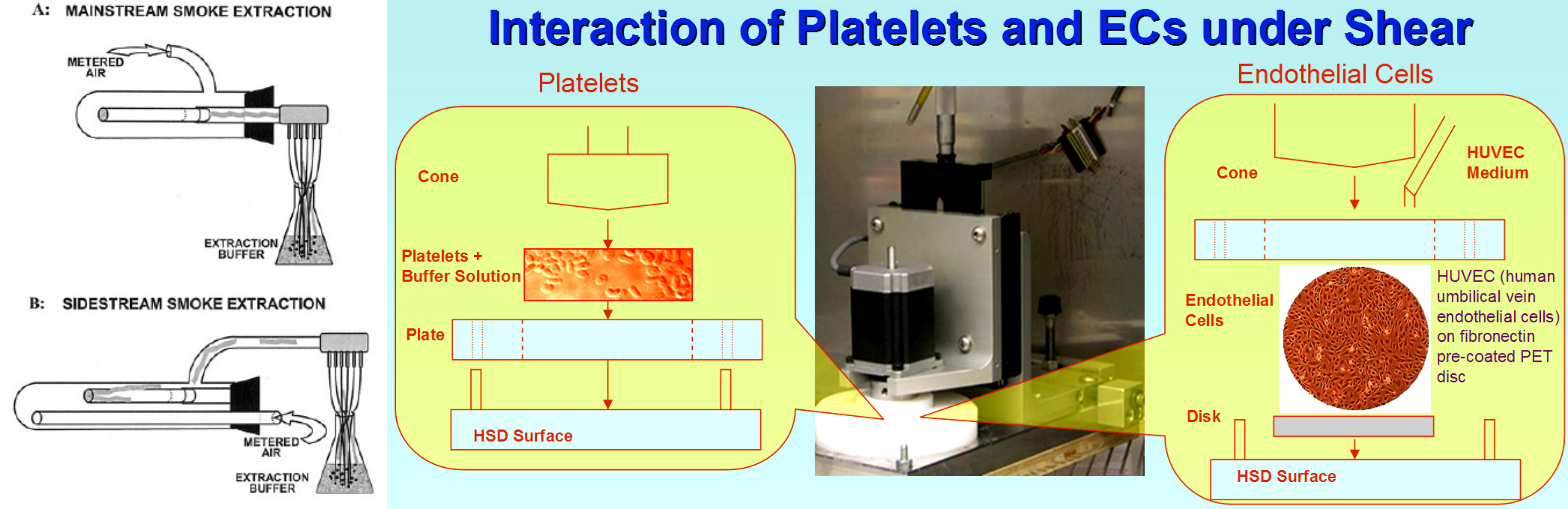
In this study, the prothrombotic properties of platelets exposed to cigarette-smoke extracts of both mainstream and sidestream type were examined, and the importance of platelet activation by these agents when exposed to flow conditions that mimic the stresses that may be encountered under normal arterial flow were described. A key focus of this study was to establish the differences between "light" (low-tar) and normal (high-tar) cigarettes. The former dilute the inhaled (mainstream) smoke with air that enters the filter through side holes and bypasses the burning tobacco. As long as these are not blocked and as long as the puff volume remains constant, these cigarettes deliver less smoke per puff.
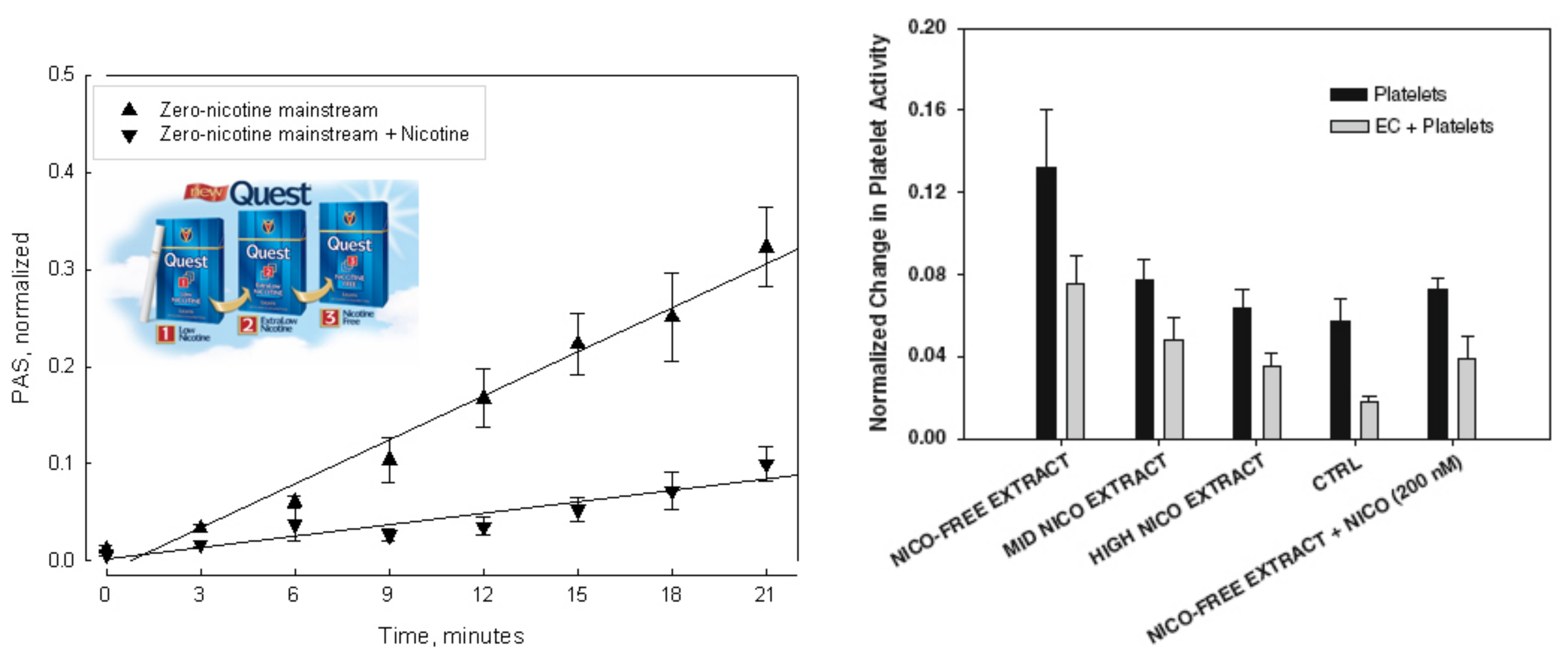
The second part of this study was devoted to discriminate between the nicotine-dependent and non-nicotine-dependent effects of tobacco smoke on platelets. The general role of platelets in cardiovascular disease associated with smoking is reasonably well established, but the role of nicotine in smoking-related cardiovascular disease has not been demonstrated. It has sometimes been tacitly assumed that the various deleterious effects of tobacco smoke are mainly caused by nicotine, but in the case of platelets there is no evidence to support this assumption, and the effects of nicotine on platelets remain unclear. In this study, the thrombogenic properties of platelets exposed to mainstream and sidestream smoke extracts from low-nicotine and zero-nicotine cigarettes under flow conditions that approximately mimic normal vascular blood flow were measured, and the effect(s) of adding nicotine to the zero-nicotine smoke extracts were determined.
The third part of this study was concerned with determining the risks associated with exposure to secondhand smoke produced by high-tar/high-nicotine, low-nicotine and zero-nicotine, and, low-tar cigarettes in individuals exposed to cigarette smoke produced by a smoking machine.
