Richard A. F. Clark, M.D.
Professor
Research Focus

Over twenty years ago, the Clark laboratory found that skin cell activation is the rate limiting step in new tissue formation of cutaneous wounds (Am J Path 149:1257-1270, 1996). The activated cells switch their cell surface receptors from those that recognize normal connective tissue, i.e. type 1 collagen, to those that recognize proteins in the provisional wound matrix, i.e. fibrin and fibronectin (FN). The cell surface integrin switch allows activated parenchymal invasion of the wound space. As a salient example, αvβ3, a fibrin integrin receptor, is expressed only on capillary sprouts invading the wound clot and is required for endothelial cell invasion of the clot (Science, 264:569-571,1994, Am J Path 148:1407-1421, 1996). In contrast, connective tissue fibroblasts do not move on pure fibrin gels and express little αvβ3. Instead, fibroblasts require FN to transmigrate from the collagen-rich connective tissue around a wound into the fibrin/FN-rich wound space (J Cell Sci, 110:861-870, 1997). To transmigrate from collagen to FN, fibroblasts switch their surface integrin receptors from collagen integrins (e.g. α2β1) to FN integrins (i.e. α5β1). This switch is driven by increased FN in the extracellular matrix, an important example of ligand positive-feedback on receptor expression (J Invest Dermatol, 113:913-919, 1999). In contrast, autocoids and growth factors induce negative-feedback on receptor expression. We have also elucidated FN domains that are critical for fibroblast transmigration (J Invest Dermatol, 121:695-705, 2003). The same FN domains were found to be necessary and sufficient to induce optimal wound healing in a porcine excisional wound model (Tiss Eng. 12:601-613, 2006, Biomaterials 28:671-679, 2007).
The years of laboratory bench and animal research on skin injury and repair provided a groundwork for development of a therapy to treat burn injury progression, a clinical unmet need. A putative therapy arose from our discovery that four sites in FN (Figure 1) bound and enhanced the ability of platelet-derived growth factor (PDGF)-BB to support fibroblast survival and growth under stress situations (J Invest Dermatol, 131:84-98, 2011). From the most robust site in the first type III repeat of FN (Figure 1), we delineated these activities to a 14-residue peptide (P12) that can also limit burn injury progression in both small and large animal burn models (J Invest Dermatol, 134:1119-1127, 2014).
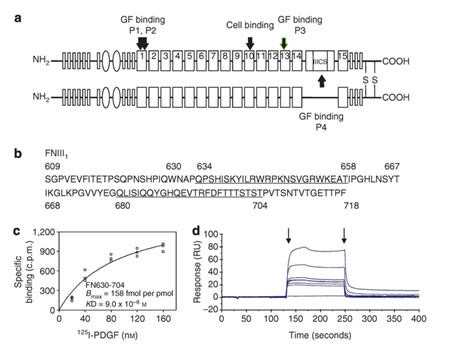
Figure 1. Peptide from first FN type III repeat (FNIII1) binds PDGF-BB. Schematic of human FN. FN type I repeats are shown as thin rectangles, FN type II repeats as ovals, and FN type III repeats as thick rectangles. PDGF-BB-binding sites (P1, P2, P3, P4) and the RGD cell-binding site are indicated with arrows. P1 contains the P12 sequence. (b) Sequence of FNIII1 (FN609-718). Sequences of P1 (FN634-658) and P2 (FN680-704) are underlined. (c) Equilibrium binding of PDGF-BB with anastellin (FN630-704). (d) Kinetic binding of PDGF-BB with FN630-704. Increasing concentrations (6.25–200 nM) of FN630-704 were injected across the biosensor chip coupled with PDGF-BB (first arrow), followed 120 seconds later by a continuing flow of buffer (second arrow). Chip without PDGF-BB was used as a reference. The dissociation constant (Kd) was calculated by averaging the koff divided by kon. Sensorgrams are representative of 3 different experiments. c.p.m., counts per minute; RU, relative units. (from J Invest Dermatol, 134:1119-1127, 2014)
Mechanistically P12 enhances PDGF-BB-induced cell survival and growth by redirecting PDGF-BB/PDGFreceptor (PDGFR) complexes from clathrin-mediated endocytosis to a macropinocytosis-like pathway, thereby augmenting Akt phosphorylation (J Invest Dermatol, 134:921-929, 2014) (Figure 2). Interestingly, while a prolongation of Akt phosphorylation (p-Akt) is observed 2 hours after addition of P12 to a physiologic serum concentration (1nM) of PDGF-BB (Figure 2, lower panel), a striking magnitude increase in p-Akt is observed 1 hour after P12 is added to wound concentrations (0.1nM or 0.3nM) of PDGF-BB (20) (Figure 2, upper panel).
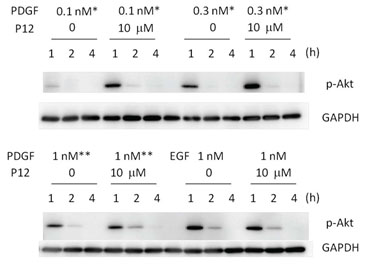
Figure 2. P12 increased p-Akt at various doses of PDGF-BB in acute wound fluid (upper panel) or PDGF-BB in serum (lower panel). Adult Human Dermal Fibroblasts (AHDFs) were treated with PDGF-BB+/-P12 in Hanks Balanced Salt Solution (HBSS) at the doses indicated in the figure. Western blots were performed with an antibody against phosphorylated-Akt (p-Akt) and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used as loading control. (from J Invest Dermatol, 134:921-929, 2014)
Preclinical efficacy studies have demonstrated that cyclized P12 (cP12) administered by infusion, 4 hours post-burn (Figure 3), improves wound healing from 30% wound closure in control animals to 70% wound closure in treated animals as judged by 14-day post-burn histomorphometry (Wd Rep Reg, 24:501-513, 2016). Furthermore, percent re-epithelization 10d post-burn is also augmented by cP12 and scar depth at 28d post-burn is reduced by cP12 (Figure 4).
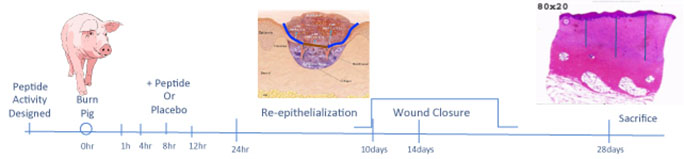
Figure 3. Discovery Paradigm Timeline. Biopsies for re-epithelialization are stained with hematoxylin and eosin (H&E) and analyzed for % re-epithelialization (re-epithelized wound surface/total wound surface x 100) by a dermatopathologist blinded to the protocol. Biopsies for scar depth are bisected, fixed and stained with H&E. Scar depth measured on H&E stained sections by three vertical lines from the epidermal-dermal junction on both bisected specimens. The six lengths are summed and divided by six to acquire the median scar depth for that specimen.
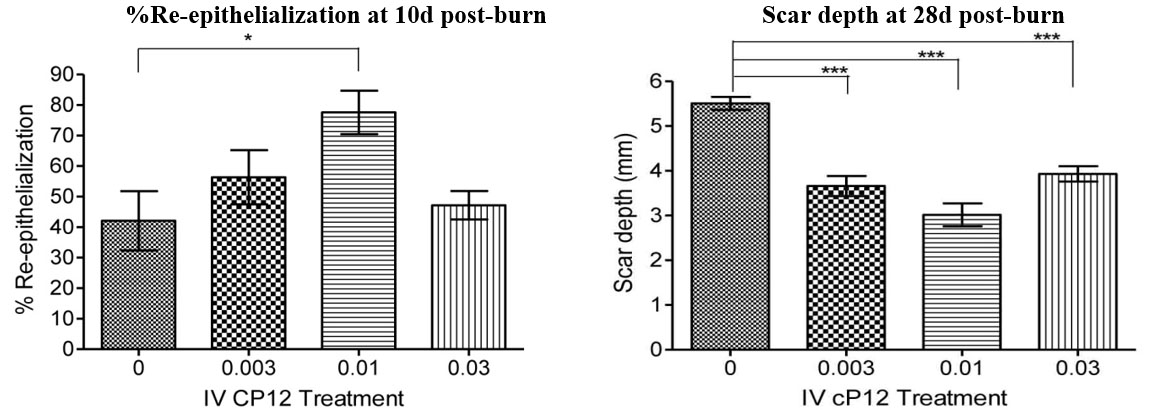
Figure 4. Cyclized P12 (cP12) infusions), speed healing, and attenuate scarring in porcine burns when administerd 4h post-burn. Percent re-epithelialization and scar depth were quantified as described in Figure 2. (from Wd Rep Reg, 24:501-513, 2016).
NeoMatrix Therapeutics, Inc. (NMT, a company founded Dr. Clark) submitted an IND August 2017, which was activated September 2017. NMT has recently submitted a proposal to begin clinical trials, entitled, “A Phase 1 Randomized, Placebo-Controlled, Single Ascending Dose Study to Examine the Safety, Tolerability, and Pharmacokinetics of cP12 in Healthy Adults.” cP12 therapy for “prevention of burn injury progression of acute, deep dermal burns in hospitalized patients" has already been given Orphan Drug Designation. Now, since cP12 treatment for burns has an active IND and is targeted to fill an unmet need of a critical medical condition, NMT will submit a FAST TRACK request to the FDA. A Phase 2a clinical trial has been outlined and with guidance from the FDA an acceptable protocol will be written.
Education
- S.B. Massachusetts Institute of Technology, Boston, MA, 1966
- M.S. Department of Microbiology, University of Rochester School of Medicine and Dentistry, Rochester, NY, 1971
- M.D. University of Rochester School of Medicine and Dentistry, Rochester, NY, 1971
Professional Experience
Internship and Residencies:
| 1971-1972 | Medical Intern, Strong Memorial Hospital, Rochester, NY |
| 1972-1973 | Medical Resident, Strong Memorial Hospital, Rochester, NY |
| 1976-1977 | Dermatology Resident, Massachusetts General Hospital, Boston, MA |
Research Fellowships:
| 1973-1976 | Clinical Associate, Laboratory of Clinical Investigation, National Institute of Allergy and Infectious Disease, National Institutes of Health |
| 1977-1978 | Dermatology Fellow, Massachusetts General Hospital, Boston, MA |
Academic Appointments:
| 1979-1981 | Instructor in Dermatology, Harvard Medical School |
| 1981-1986 | Assistant Professor in Medicine and Dermatology, University of Colorado Health Sciences Center |
| 1986-1990 | Associate Professor in Dermatology and Medicine, University of Colorado Health Sciences Center |
| 1990- | Professor, Department of Dermatology, Stony Brook University |
| 1990-2003 | Chair, Department of Dermatology, Stony Brook University |
| 2000-2003 | Adjunct Professor, Department of Bioengineering, Stony Brook University |
| 2001- | Adjunct Professor of Medicine, Stony Brook University |
| 2003- | Professor of Biomedical Engineering, Stony Brook University |
| 2003- | Director, Center for Tissue Engineering |
Hospital Appointments:
| 1979-1981 | Clinical Associate in Dermatology, Massachusetts General Hospital, Boston, MA |
| 1980-1981 | Research Associate in Pathology, Beth Israel Hospital, Boston, MA |
| 1980-1981 | Assistant in Dermatology, Beth Israel Hospital, Boston, MA |
| 1981-1988 | Member, Division of Clinical Immunology, Department of Medicine, National Jewish Center, Denver, CO |
| 1986-1988 | Director, Dermatology Program, Departments of Medicine and Pediatrics, National Jewish Center, Denver, CO |
| 1988-1990 | Head, Division of Dermatology, Departments of Medicine and Pediatrics, National Jewish Center, Denver, CO |
| 1990-2003 | Chief of Service, Dermatology, University Hospital, Stony Brook University |
| 2003- | Member, Dermatology Service, University Hospital, Stony Brook University |
Honors and Awards
| 1962-1966 | Store Scholarship, Massachusetts Institute of Technology |
| 1966-1968 | Alumni Scholarship, University of Rochester School of Medicine and Dentistry |
| 1968-1971 | Clinical Investigator Training Fellowship |
| 1970 | Election to Alpha Omega Alpha |
| 1971 | Distinction in Research, University of Rochester School of Medicine & Dentistry |
| 1975 | American Academy of Allergy Travel Grant |
| 1979 | Society for Investigative Dermatology Award |
| 1979 | Boston Medical Foundation Award |
| 1989 | Election to the American Society for Clinical Investigation |
| 1993 | Election to the American Dermatology Association |
| 1995 | Teaching Award, Department of Dermatology, Stony Brook University |
| 1996 | NIH Merit Award |
| 1997 | Election to the New York Dermatology Society |
| 1999 | Election to the American Association of Physicians |
| 1999 | Elected Fellow, American Association for the Advancement of Science |
| 2001 | Program Excellence Award , Department of Dermatology, Stony Brook University |
| 2002 | Scholar, Harvard-Macy Institute, Leadership in Education |
| 2006 | President's Award, Distinguished PhD Student, Kaustabh Ghosh (Thesis Advisor) |
| 2009 | President's Award, Distinguished PhD Student, Zhi Pan (co-Thesis Advisor) |
| 2011 | Senior Author - Best Poster, American Burn Association Annual Meeting, 1st author, Doug Hirth, 2nd year Med Student |
| 2012 | Teaching Award, Department of Biomedical Engineering, Stony Brook University |
| 2013 | Senior Author Finalist, Excellence in Translational Research, Wound Healing Society Annual Meeting, 1st author, Jia Zhu, 5th year Graduate Student |
| 2015 | Elected Honorary Member of the Society for Investigative Dermatology |
| 2015 | Service Award, Department of Dermatology |
| 2016 | Elected to Stony Brook University Chapter of National Academy of Inventors |
| 2017 | First Place Award: Industrial Research & Development, Wound Healing Annual Meeting, San Diego, CA, April 5-8, 2017 |
Professional Activities and Affiliations
Licensure and Certifications:
| 1972 | National Board of Medical Examiners |
| 1974 | American Board of Internal Medicine |
| 1977 | American Board of Allergy and Clinical Immunology |
| 1980 | American Board of Dermatology |
| 1981 | State of Colorado |
| 1990 | State of New York |
Professional Society Memberships:
| 1977 | Dermatology Foundation (DF) |
| 1979 | American Association for the Advancement of Science (AAAS) |
| 1979 | American Federation for Clinical Research (AFCR) |
| 1980 | Society for Investigative Dermatology (SID) |
| 1981 | American Academy of Dermatology (AAD) |
| 1983 | American Academy of Allergy and Immunology (AAAI) |
| 1983 | American Society of Cell Biology (ASCB) |
| 1989 | American Society for Clinical Investigation (ASCI) |
| 1990 | Wound Healing Society (WHS) |
| 1990 | Long Island Dermatology Society |
| 1990 | Association of Professors of Dermatology (APD) |
| 1991 | Suffolk County Dermatology Society |
| 1993 | American Dermatology Association (ADA) |
| 1994 | New York Academy of Medicine, Dermatology Section |
| 1997 | New York Dermatology Society |
| 1999 | American Association of Physicians (AAP) |
| 2000 | American Association of Medical Colleges (AAMC) |
| 2004 | Tissue Engineering Society International (TESI) |
Professional Society, NIH, Editorial Board Offices and Committee Assignments:
| 1989- | Scientific Board, Eczema Association for Science and Education |
| 1990-1997 | Associate Editor, J Cell Biochem |
| 1990-1994 | Board of Directors, WHS |
| 1990-1996 | Chair of Ethics Committee, WHS |
| 1990-1994 | Chair, AAD and AAAI Liaison Task Force |
| 1991-1994 | Scientific Board of Advisors, Curative Technologies, Inc. |
| 1992-1994 | Program Committee, AAAI |
| 1992-1996 | Member, NIA Liaison Committee, AAD |
| 1993-1996 | Chair of Government Relations Committee, WHS |
| 1993-2000 | Vice-Chair, Leader's Society, Dermatology Foundation |
| 1993-1996 | Medical and Scientific Committee, Dermatology Foundation |
| 1993-1994 | Chair, Dermatology Disease Section, AAAI |
| 1994-1995 | Secretary, New York Academy of Medicine, Dermatology Section |
| 1994- | Scientific Board, Dystrophic Epidermolysis Bullosa Research Association |
| 1995-1996 | President, New York Academy of Medicine, Dermatology Section |
| 1995-1996 | President-elect, WHS |
| 1995-1999 | Executive Committee, WHS |
| 1995-1999 | NIAMS Special Grants Review Committee |
| 1996-1997 | President, WHS |
| 1996-1999 | Scientific Board of Advisors, Vitex, Inc. |
| 1997-1999 | Vice-President, WHS |
| 1997-1999 | Chair, Nominations Committee, WHS |
| 1997-2000 | Chair, Government Relations Committee, WHS |
| 1997- | Institutional Representative , ASCI |
| 1997-2002 | Member, Government and Public Relations Committee, SID |
| 1998-2000 | Chair, Government and Public Relations Committee, SID |
| 1998-1999 | Member, Publications Committee, SID |
| 1999-2003 | Board of Directors, APD |
| 2000- | Representative , Academic Societies (CAS), AAMC |
| 2001-2005 | Chair, NIA Liaison Task Force, AAD |
| 2001-2005 | Member, Research Committee, AAD |
| 2001-2006 | Patient Advocate Liaison, SID |
| 2002-2006 | Chair, Patient Advocate Task Force, AAD |
| 2002-2006 | Member, Government Affairs Committee, AAD |
| 2002-2006 | Member, Research Committee, AAD |
| 2002-2004 | Treasurer , Long Island Dermatology Society |
| 2002-2008 | President-elect, Long Island Dermatology Society |
| 2002-2007 | Associate Editor, Journal of Investigative Dermatology |
| 2002 | Selection Committee for the AOA Glaser Teaching Award, AAMC |
| 2002-2007 | Member, Scientific Advisory Committee, Ad Hoc Group for Medical Research Funding, AAMC |
| 2003-2007 | Member, Board of Directors, Society for Investigative Dermatology |
| 2003 | Cutaneous Biology Core Center, Ad Hoc Study Section |
| 2004-2009 | Member, Long Range Planning Committee, Society for Investigative Dermatology |
| 2004-2006 | Secretary, Long Island Dermatology Society |
| 2004 | Member, NIAMS Skin Branch Long Range Planning Committee |
| 2004 | External Advisory Board Member, Cleveland Clinic, Depart. Biomedical Engineering |
| 2005-2009 | Member, Research Committee, AAD |
| 2006 | Scientific Advisory Board Member, Clinical Tissue Engineering Center, Cleveland, OH |
| 2006-2008 | Vice-President, Long Island Dermatology Society |
| 2006-2008 | Scientific Advisory Board Member, Omrix, Biopharmaceuticals, Ltd |
| 2007 | NIAMS Special Emphasis Panel (MOSS) Study Section |
| 2007-2017 | Wound Healing and Regeneration Section Editor, Journal of Investigative Dermatology |
| 2008-2010 | President, Long Island Dermatology Society |
| 2008 | NIAMS Special Emphasis Panel (MOSS) Study Section |
| 2008 | DOD Review Panel, Deployment Related Medical Research Program (DRMRP) |
| 2008-2009 | President-elect, Society for Investigative Dermatology |
| 2008-2013 | Director: Skin, Burn and Nonscar Healing Program, Rutgers-Cleveland Clinic Consortium (RCCC), Armed Forces Institute of Regenerative Medicine (AFIRM) |
| 2009-2010 | President, Society for Investigative Dermatology |
| 2009-2010 | President, New York Dermatology Society |
| 2009-2017 | Scientific Board of Advisors, RESBIO, Rutgers University |
| 2009 | NIAMS Special Emphasis Panel (MOSS) for Challenge Grant Reviews |
| 2010 | NIAMS Ancillary Studies Panel for P50 proposals |
| 2010-2011 | Immediate Past-President, Society for Investigative Dermatology |
| 2010-2011 | Member, Board of Directors, Society for Investigative Dermatology |
| 2010-2011 | Chair, Search Committee for Secretary-Treasurer, Society for Investigative Dermatology |
| 2010-2013 | Member, Nominations Committee, Society for Investigative Dermatology |
| 2011 | External Advisor, Skin Disease Research Center, University of Alabama, Birmingham |
| 2012 | NIAMS Ancillary Studies Panel for P50 proposals |
| 2013 | Chair, Nominations Committee, Society for Investigative Dermatology |
| 2013-2018 | co-Focus Leader, Skin Regeneration, Armed Forces Institute of Regenerative Medicine II |
| 2014 | Chair, Biomedical Engineering Faculty Search Committee |
| 2014- | Chair, Executive Committee, Biomedical Engineering |
| 2015-2017 | President, School of Medicine Faculty Senate |
Publications
Publications via Google Scholar
Patents
- US Patent 5,958,874 Recombinant fibronectin-based extracellular matrix for wound healing, September 28, 1999.
- US Patent 6,194,378 Fibronectin peptides-based extracellular matrix for wound healing, February 27, 2001.
- US Patent 6,268,215 Recombinant keratinocytes, July 31, 2001.
- US Patent 6,723,302 Model for cell migration and use thereof, April 20, 2004.
- US Patent 6,946,140 Methods and compositions for enhancing fibroblast migration, Sept 20, 2005.
- US Patent 8,691,944 Fibronectin polypeptides and uses thereof. April 8, 2014.
- US Patent 8,759,300 Fibronectin polypeptides and uses thereof. June 24, 2014
Courses Taught
- BME 404 - Essentials of Tissue Engineering
