Faculty
Stanislaus S. Wong, Distinguished Professor & Chair

B.Sc., 1994, McGill University
A.M., 1996, Harvard University
Ph.D., 1999, Harvard University
Postdoctoral fellow, 1999-2000, Columbia University
415 Chemistry
Phone: (631) 632-1703 | BNL Phone: (631) 344-3178
Email: Stanislaus.Wong@stonybrook.edu | sswong@bnl.gov
Positions and roles
- Chair (September 2023 - present) of the Chemistry Department at SUNY Stony Brook.
- Assistant Professor (2000-2006), Associate Professor (2006-2010), Professor (2010-2019), and Distinguished Professor (2019-present) of Chemistry at the State University of New York at Stony Brook.
- Vice Chair (June 2019 - August 2023) for Facilities, Budget, and Research in the Chemistry Department at SUNY Stony Brook.
- Joint appointment (2000-2017) with the Condensed Matter Physics and Materials Sciences Department at Brookhaven National Laboratory. Affiliated member of the Biomedical Engineering Program and the Biophysics Program at the State University of New York at Stony Brook.
- Executive Editor (August 28, 2018 – present) and Associate Editor (January 1, 2014 – present) of ACS Applied Materials & Interfaces.
- Member of the Editorial Advisory Board of Chemistry of Materials (2008 - 2013).and of ACS Applied Materials and Interfaces (2013).
- Section editor for the ‘Materials: synthesis and self-assembly’ section of Nanotechnology (2010-2013) and current member of editorial advisory boards of both Nanotechnology (2010 – present) and of Nano Futures (2017 – present).
- Nanoscience Chair-Elect (2008), Nanoscience Chair (2009), and Nanoscience Programming Chair (2009-2012) of the Inorganic Chemistry Division (American Chemical Society).
- Scientific advisory board member of the Association of Students and Postdoctoral Fellows (ASAP) at Brookhaven National Laboratory (2010 - 2017).
- Member of the Institutional Nanoscience Safety Advisory Committee at Brookhaven National Laboratory (2006 - 2014).
- Chair of the Center for Functional Nanomaterials (CFN) User Executive Committee at BNL (2009–2010; 2013-2014; and 2016-2017).
- Co-organizer of the following joint National Synchrotron Light Source (NSLS) / CFN Users’ Meetings: (a) 2010 - “Understanding and Mitigating the Effects of Climate Change Using Synchrotron and Nanoscience Research”; (b) 2013 - “Telling our Story, Sharing our Science”; and (c) 2016 - “Illuminating our Future”.
- Guest co-editor (with Barbara Karn), ‘Green Nanotechnology’, Special issue, Nanotechnology, volume 23, issue 29, July 27, 2012. Special issue papers 294001 through 294014.
- Guest co-editor (with Taeghwan Hyeon and Liberato Manna). Special themed collection on “Sustainable Nanotechnology”, Chemical Society Reviews, volume 44, issue 16, August 21, 2015.
- Member of the Materials Research Society National Nanotechnology Initiative Task Force (Summer 2010).
Research Description
I. Global Directions and Themes
My group’s research has primarily focused on two main areas (namely, nanotube chemistry and nanostructure synthesis) that will broaden the potential impact and practical applicability of nanostructures.
A. Carbon Nanotube (CNT) Functionalization
In this area, we have reacted nanotubes as if there were chemical ligands (be it inorganic or organic) in their own right, e.g. as if these were simply complex alkenes. The work in our laboratory has been involved with understanding chemical reactivity involving carbon nanotubes from a structural and mechanistic perspective, which should hopefully expand the breadth and types of reactions CNTs can undergo in the solution phase. Specifically, the protocols that we have created have significantly enhanced the ability to purify, exfoliate, process, solubilize, and even render biocompatible CNTs, thereby permitting more facile photophysical, catalytic, and biomedical applications of these systems. Controllable chemical functionalization suggests that the unique optoelectronic and mechanical properties of SWNTs can be tailored in a determinable manner.
Key Case Studies of Successful Carbon Nanotube Functionalization in Inorganic, Organic, and Biological Systems
(i). Inorganic Systems
(ii). Organic Systems
(iii). Biological Systems
B. Green Nanostructure Synthesis
Early on, we expended a lot of effort on developing innovative syntheses of nanoscale formulations (including cubes, tubes, wires, spheres, and rhombohedra as well as aggregates and arrays) of perovskite oxide materials. More generally, we have implemented a number of viable environmentally friendly synthetic methodologies in the fabrication of a range of ternary and binary metal oxides, elemental metals, titanates, fluorides, phosphates, sulfides, tungstates, niobates, zirconates, ruthenates, and ferrites. In fact, most of our processes run under either ambient conditions or low temperatures, and can be efficiently scaled up. Moreover, our simple protocols are generally cost-effective; use mainly nontoxic precursors; limit the numbers of reagents and reaction steps; minimize waste, reagent use, and power consumption; and involve the development of high-yield processes with a relative absence of volatile and toxic byproducts.
In particular, we have made important advances in the use of molten-salt synthetic methods, hydrothermal protocols, and ambient template-directed techniques as green, cost-effective methodologies to generate monodisperse nanostructures with precise size and shape control without sacrificing on sample quality, purity, and crystallinity. Our as-prepared nanomaterials maintain fundamentally interesting size-dependent electronic, optical, and magnetic properties. In terms of applications, these nanostructures have wide-ranging utility in areas as diverse as catalysis, energy storage, biomedicine, computation, power generation, photonics, remediation, and sensing.
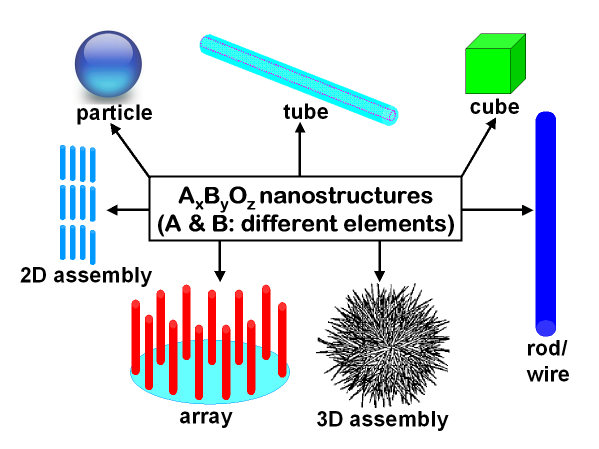
Key Case Studies of Successful Metal-Containing Nanostructure Synthesis
(i). Tungstates
(ii). Fluorides
(iii). Magnetic Nanostructures
(iv). Perovskite Nanostructures
(v). Titanate Nanostructures
(vi). Binary Systems
II. New and Emerging Trends
A. Fuel Cells
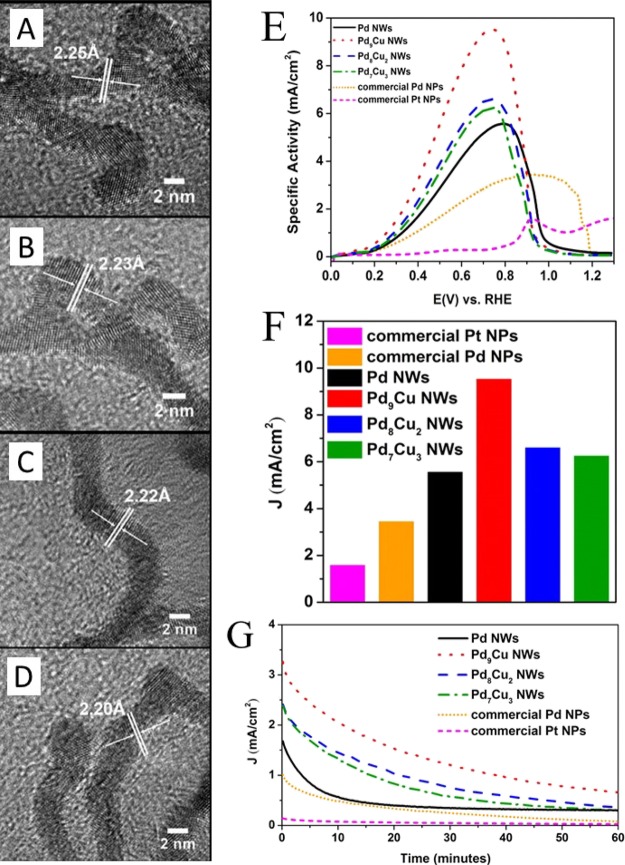
Currently, Pt-based catalysts still represent the most widely used catalysts for applications in fuel cells due to their high activity. Nevertheless, we have initiated a number of efforts towards optimizing (1) the intrinsic particle size of Pt nanostructures, (2) the production of novel morphological motifs, such as either nanowires or three-dimensional nanostructures, as well as (3) the spatial distribution and dispersion of these nanoparticles to transcend the traditional view of elemental Pt itself as the most important and best performing fuel cell catalyst. This work exploring a range of alternatives to conventional catalysts and support media is summarized in a number of invited contributions including ACS Applied Materials and Interfaces, v.13, 58253 (2021); Chem. Eur. J., v.25, 7779 (2019); ACS Omega, v.3, 3294 (2018); APL Materials, v.3, 080701 (2015); Chem. Soc. Rev., v.44, 5836 (2015); J. Phys. Chem. Lett., v.3, 3385 (2012); and Energy & Environmental Sciences,v.4, 1161 (2011). The scope of the novelty of our contributions is covered by issued U.S. patents #9,440,290 B2; #9,490,486; #9,624,598; and #9,623,481. As an example, some of the highest electrochemical activities reported to date have been obtained by deliberately tuning the size, morphology, and chemical compositions of Pt-based alloyed nanostructures. In so doing, activities of over a magnitude higher than the state-of-the-art commercial Pt catalyst have been achieved.
We and other groups have explored a number of nanowire-based catalytic architectures, composed of Pt-free or reduced Pt content, as an energy efficient solution with improved performance metrics versus conventional, currently commercially available Pt nanoparticles that are already well known in the community. Our goal therefore has been to precisely, synergistically, and simultaneously tune size, morphology, architectural motif, surface chemistry, and chemical composition of generated catalysts coupled with the nature of the underlying support so as to controllably improve performance metrics of the hydrogen oxygen reaction (HOR), the methanol oxidation reaction (MOR), the ethanol oxidation reaction (EOR), the formic acid oxidation reaction (FAOR), in addition to the oxygen reduction reaction (ORR). As such, we have developed ambient synthetic methods to prepare morphology and composition-tunable, crystalline one-dimensional nanostructures possessing reproducible structural uniformity and a homogeneous distribution of elements on a large and sustainable scale. These advances are of relevance for creating a cheaper, more durable, more efficient, high-performing, and distinctive family of catalytic materials that will also be inherently less susceptible to poisoning and degradation.
B. Solar Cells
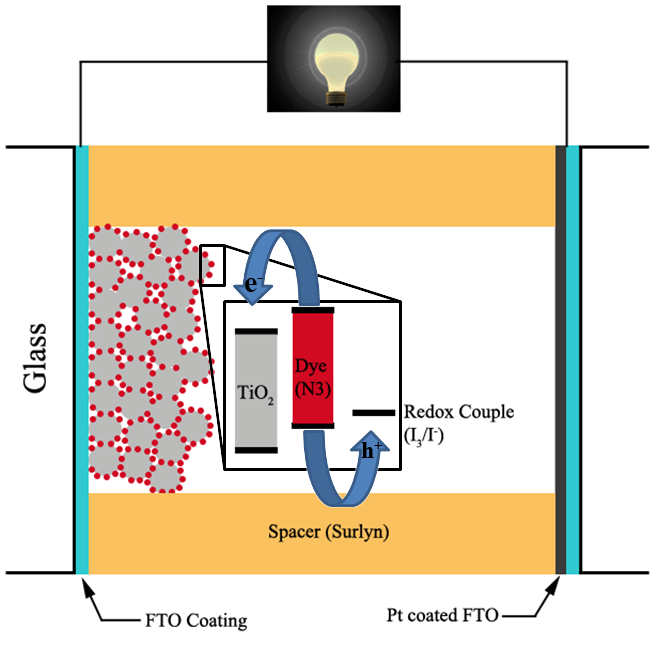
The ability to synthesize, functionalize, and purify nanomaterials and to understand their transport, optical and mechanical properties lies at the forefront of current materials science research. Nanostructured materials offer several potential advantages for solar energy conversion by providing relatively high electron and hole transport efficiencies, excitation multiplication possibilities, high surface-to-volume ratios, and short electron-hole diffusion lengths to junctions. However, the role of nanoscale heterojunctions in promoting exciton dissociation and charge separation is not understood. It is expected that junctions between low dimensional materials will behave differently as compared with bulk junctions. We addressed the important question of the efficiency of charge separation versus recombination or trapping in a prototype heterogeneous nanomaterial system, i.e. 0d-1d nanomaterial hybrids. We also developed a fundamental emphasis on synthesizing and understanding more complex nanoscale materials, including metallic heterostructures, of significance to the solar initiative. We had the ability to synthesize high quality samples and to study the properties and structure (both electronic and physical) of individual nanomaterial samples, thus eliminating the uncertainties in ensemble averaging over many structures. Along with theory, these studies clarified the potential of nanoscale heterojunctions for charge separation in solar electric generation. Our activities have been summarized in a number of papers including Energy & Environmental Sciences, v.12, 1454 (2019) and Chem. Soc. Rev., v.42, 8134 (2013).
Nanotube-Nanocrystal Heterostructures
C. Battery-related applications
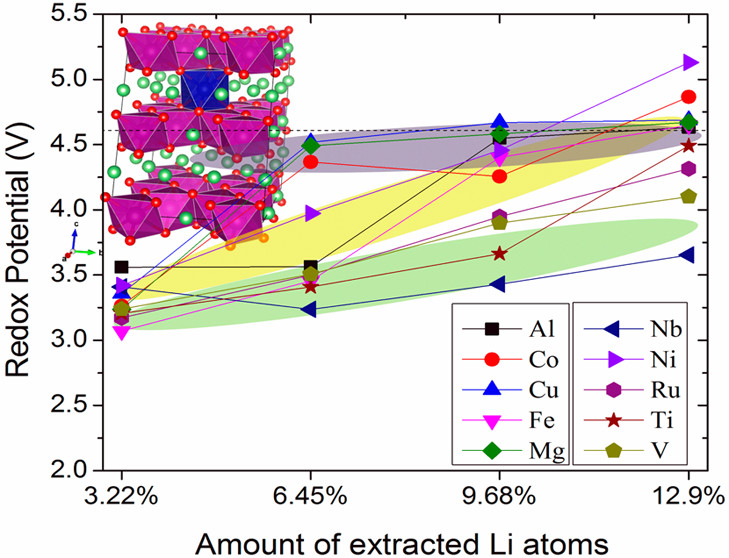
In a parallel effort (ACS Energy Letters, v.2, 1465 (2017) and Nanomaterials, v.13, 1599 (2023)), we have noted that metal oxides and sulfides represent a set of promising materials for use as electrodes within lithium ion batteries but unfortunately, these tend to suffer from limitations associated with poor ionic and electron conductivity as well as low cycling performance. Hence, to achieve the goal of creating economical, relatively less toxic, thermally stable, and simultaneously high-energy-density electrode materials, we have put forth and analyzed a number of targeted strategies, aimed at rationally improving upon electrochemical performance. Specifically, we have carefully studied the precise roles and effects of controllably varying not only (i) morphology but also (ii) chemistry as a means of advancing, ameliorating, and fundamentally tuning the development and evolution of systems such as FeS2, Fe3S4, Fe3O4, Li4Ti5O12, TiO2, and LiV3O8 as viable and ubiquitous energy storage materials.
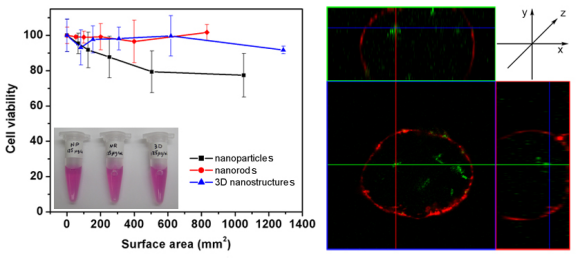
D. Nuances of Nanomaterial Toxicology
The use of any material for practical applications engenders risk. Frankly, even water itself can be fatal if misused. What is important is in understanding what constitutes acceptable risk. For nanomaterials, the key point is in determining whether a substance is inherently toxic and under what specific circumstances, it can be particularly harmful. In both cellular and marine studies, our group has found that the toxicity of a nanomaterial is often a function not only of its actual chemical composition but also of its particular structural morphology.
Nanostructures for Biological Labeling
Representative Perspective, Review, and Concept Articles
Leela Sotsky, Angeline Castillo, Hugo Ramos, Eric Mitchko, Joshua Heuvel-Horwitz, Brian Bick, Devinder Mahajan, and Stanislaus S. Wong, “Hydrogen Storage Properties of Metal-Modified Graphene Materials”, invited contribution, Energies, 17, 3944 / 1-25 (2024).
Kenna L. Salvatore, Justin Fang, Christopher R. Tang, Esther S. Takeuchi, Amy C. Marschilok, Kenneth J. Takeuchi, and Stanislaus S. Wong, “Microwave-Assisted Fabrication of High Energy Density Binary Metal Sulfides for Enhanced Performance in Battery Applications”, invited Review, Nanomaterials, 13, 1599 / 1-20 (2023).
Nathaniel Hurley, Scott C. McGuire, and Stanislaus S. Wong, “Assessing the Catalytic Behavior of Ultrathin Nanowires Using X-ray Absorption Spectroscopy”, invited contribution, ACS Applied Materials and Interfaces, 13(49), 58253−58260 (2021).
Stanislaus S. Wong, “Sustainable synthesis of functional nanomaterials” in “Voices: Sustainable opportunities for critical metals”, invited contribution, One Earth, 4(3), 327-330 (2021).
Kenna L. Salvatore and Stanislaus S. Wong, “Exploring Strategies Towards Synthetic Precision Control within Core-shell Nanowires”, invited contribution, Accounts of Chemical Research, 54(11), 2565−2578 (2021).
Luyao Li, Sha Tan, Kenna L. Salvatore, and Stanislaus S. Wong, “Nanoscale Perovskites as Catalysts and Supports for Direct Methanol Fuel Cells”, invited contribution with Frontispiece, Chem. Eur. J., 25(33), 7779-7797 (2019).
Shiyu Yue, Luyao Li, Scott C. McGuire, Nathaniel Hurley, and Stanislaus S. Wong, “Quantum Dot-Sensitized 1D-based Heterostructures for Optical-related Applications”, invited Perspective, Energy & Environmental Sciences, 12(5), 1454-1494 (2019).
Luyao Li and Stanislaus S. Wong, “Ultrathin Metallic Nanowire-based Architectures as High-Performing Electrocatalysts”, invited Perspective, ACS Omega,3(3), 3294−3313 (2018).
Lei Wang, Shiyu Yue,Qing Zhang, Yiman Zhang, Yue Ru Li, Crystal S. Lewis,Kenneth J. Takeuchi, Amy C. Marschilok, Esther S. Takeuchi, and Stanislaus S. Wong, “Morphological and chemical tuning of high-energy density metal oxides for lithium ion battery electrode applications”, invited Perspective,ACS Energy Letters,2(6), 1465−1478 (2017).
Haiqing Liu, Luyao Li, Megan E. Scofield, and Stanislaus S. Wong, “Synthesis, Properties, and Applications of Ultrathin Metallic Nanowires and Associated Heterostructures”, invited Research Update, APL Materials,3(8), 080701/1-15 (2015).
Megan E. Scofield, Haiqing Liu, and Stanislaus S. Wong, “A Concise Guide to Sustainable PEMFCs: Recent Advances in Improving both Oxygen Reduction Catalysts and Proton Exchange Membranes”, invited Critical Review, Chem. Soc. Rev.(inside cover), 44(16), 5836-5860 (2015).
Lei Wang, Haiqing Liu, Robert M. Konik, James A. Misewich, and Stanislaus S. Wong, “Carbon Nanotube-based Heterostructures for Solar Energy Applications”, invited Critical Review, Chem. Soc. Rev., 42(20), 8134 – 8156 (2013).
Christopher Koenigsmann, Megan E. Scofield, Haiqing Liu, and Stanislaus S. Wong, “Designing Enhanced One-Dimensional Electrocatalysts for the Oxygen Reduction Reaction: Probing Size-and Composition-Dependent Electrocatalytic Behavior in Noble Metal Nanowires”, invited Perspective, J. Phys. Chem. Lett. (cover), 3(22), 3385-3398 (2012). Featured in “The Magic of Electrocatalysts” editorial (J. Phys. Chem. Lett., 3(22), 3404 (2012)) and highlighted in accompanying Perspective video located at http://pubs.acs.org/page/jpclcd/wong.html.
Christopher Koenigsmann and Stanislaus S. Wong, “One-Dimensional Noble Metal Electrocatalysts: A New Structural Paradigm for Direct Methanol Fuel Cells”, invited Perspective article, Energy & Environmental Sciences (cover), 4(4), 1161 – 1176 (2011).
Jonathan M. Patete, Xiaohui Peng, Christopher Koenigsmann, Yan Xu, Barbara Karn, and Stanislaus S. Wong, invited critical review, “Viable methodologies for the Synthesis of High-Quality Nanostructures”, Green Chemistry, 13(3), 482-519 (2011). One of the top 10 accessed articles in Green Chemistry in March, April, and June 2011.
Amanda L. Tiano, Alexander C. Santulli, Christopher Koenigsmann, and Stanislaus S. Wong,“Solution-Based Synthetic Strategies for One-Dimensional Metal-Containing Nanostructures”, invited Feature Article, Chem. Commun., 46(43), 8093-8130 (2010).
Xiaohui Peng, Jingyi Chen, James A. Misewich, and Stanislaus S. Wong, “Carbon Nanotube-Nanocrystal Heterostructures”, invited Critical Review, Chem. Soc. Rev. (inside cover), 38(4), 1076-1098 (2009).
Xiaohui Peng and Stanislaus S. Wong, “Functional Covalent Chemistry of Carbon Nanotube Surfaces”, invited Progress Report, Adv. Mater., 21(6), 625-642 (2009).
Yuanbing Mao, Tae-Jin Park, Fen Zhang, Hongjun Zhou, and Stanislaus S. Wong; “Environmentally friendly methodologies of nanostructure synthesis”, invited review, Small, 3(7), 1122-1139 (2007).
Tae-Jin Park, Sarbajit Banerjee, Tirandai Hemraj-Benny, and Stanislaus S. Wong, “Purification Strategies and Purity Evaluation Techniques for Single-Walled Carbon Nanotubes”, J. Mater. Chem. (Feature Article; cover), 16(2), 141-154 (2006).
Tirandai Hemraj-Benny, Sarbajit Banerjee, Sharadha Sambasivan, Mahalingam Balasubramanian, Daniel A. Fischer, Gyula Eres, Alexander A. Puretzky, David B. Geohegan, Douglas H. Lowndes, Weiqiang Han, James A. Misewich, and Stanislaus S. Wong, “Near-edge X-ray Absorption Fine Structure Spectroscopy as a Tool for Investigating Nanomaterials”, Small (Concepts Article), 2(1), 26-35 (2006).
Yuanbing Mao, Tae-Jin Park, and Stanislaus S. Wong, “Synthesis of classes of ternary metal oxide nanostructures”, Chem. Commun. (Invited Feature Article; inside cover), (46), 5721-5735 (2005).
Sarbajit Banerjee, Tirandai Hemraj-Benny, and Stanislaus S. Wong, “Routes Towards Separating Metallic and Semiconducting Nanotubes”, invited review, J. Nanosci. Nanotech., 5(6), 841-855 (2005).
Sarbajit Banerjee, Tirandai Hemraj-Benny, and Stanislaus S. Wong, “Covalent Surface Chemistry of Single-Walled Carbon Nanotubes”, invited review, Adv. Mater., 17(1), 17-29 (2005).
Sarbajit Banerjee, Michael G.C. Kahn, and Stanislaus S. Wong, “Rational Chemical Strategies for Carbon Nanotube Functionalization”, invited Concepts article, Chem. Eur. J., 9(9), 1898-1908 (2003).
