Nanomaterials for Batteries
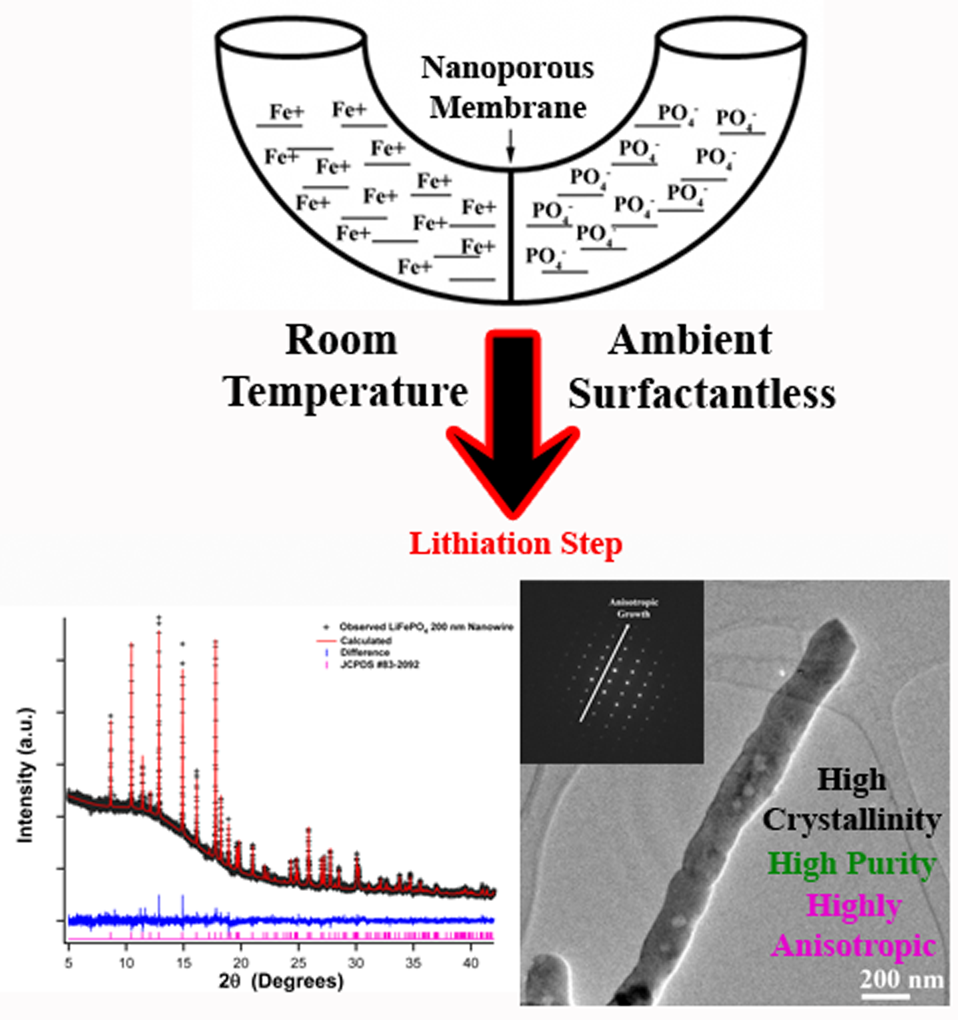
1. LiFePO4 materials have become increasingly popular as a cathode material due to the many benefits they possess including thermal stability, durability, low cost, and long life span. Nevertheless, to broaden the general appeal of this material for practical electrochemical applications, it would be useful to develop a relatively mild, reasonably simple synthesis method of this cathode material. Herein, we describe a generalizable, 2-step methodology of sustainably synthesizing LiFePO4 by incorporating a template-based, ambient, surfactantless, seedless, U-tube protocol in order to generate size and morphologically tailored, crystalline, phase-pure nanowires. The purity, composition, crystallinity, and intrinsic quality of these wires were systematically assessed using TEM, HRTEM, SEM, XRD, SAED, EDAX, and high-resolution synchrotron XRD. From these techniques, we were able to determine that there is an absence of defects present in our wires, supporting the viability of our synthetic approach. Electrochemical analysis was also employed to assess their electrochemical activity. Although our nanowires do not contain any noticeable impurities, we attribute their less than optimal electrochemical rigor to differences in the chemical bonding between our LiFePO4 nanowires and their bulk-like counterparts. Specifically, we demonstrate for the first time experimentally that the Fe-O3 chemical bond plays an important role in determining the overall conductivity of the material, an assertion which is further supported by recent first principle calculations. Nonetheless, our ambient, solution-based synthesis technique is capable of generating highly crystalline and phase-pure energy-storage-relevant nanowires that can be tailored so as to fabricate different sized materials of reproducible, reliable morphology.
Ref.: Nano Research, v.8(8), 2573-2594 (2015).
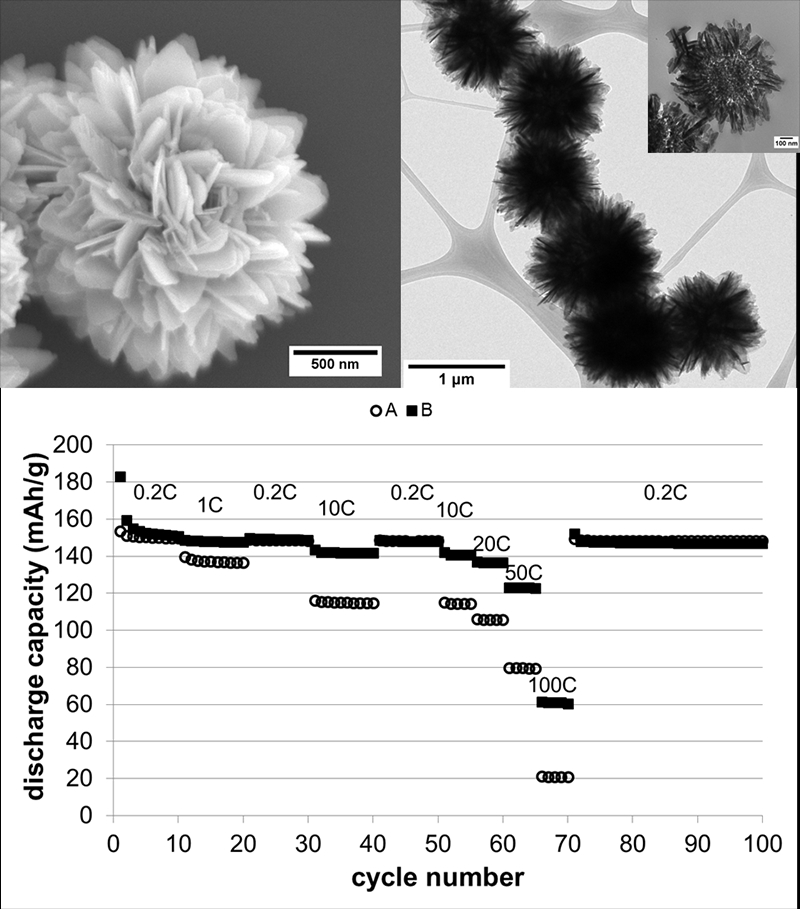
2. ‘Flower-like’ motifs of Li4Ti5O12 have been synthesized by using a facile and large-scale hydrothermal process involving unique Ti foil precursors followed by a short, relatively low-temperature calcination in air. Moreover, a detailed time-dependent growth mechanism as well as a reasonable reaction scheme have been proposed to clearly illustrate and highlight the structural evolution and subsequent formation of this material. Specifically, the resulting ‘flower-like’ Li4Ti5O12 microspheres consisting of thin nanosheet constituents can provide for an enhanced surface area and a reduced lithium ion diffusion distance. The high surface areas of the exposed roughened, thin petal-like component nanosheets are beneficial for the interaction of the electrolyte with Li4Ti5O12, thereby ultimately providing for improved high-rate performances and favorable charge/discharge dynamics. Electrochemical studies of the as-prepared nanostructured Li4Ti5O12 clearly reveal their promising potential as an enhanced anode material for lithium ion batteries, thereby presenting both excellent rate capabilities (delivering 148, 141, 137, 123, and 60 mAh g-1 under discharge rates of 0.2 C, 10 C, 20 C, 50 C, and 100 C, at cycles of 50, 55, 60, 65, and 70, respectively) and stable cycling performance (exhibiting a capacity retention of ~97% from cycles 10-100, under a discharge rate of 0.2 C, and an impressive capacity retention of ~87% using a more rigorous discharge rate of 20 C from cycles 101-300).
Ref.:ChemSusChem, v.8(19), 3304-3313 (2015).
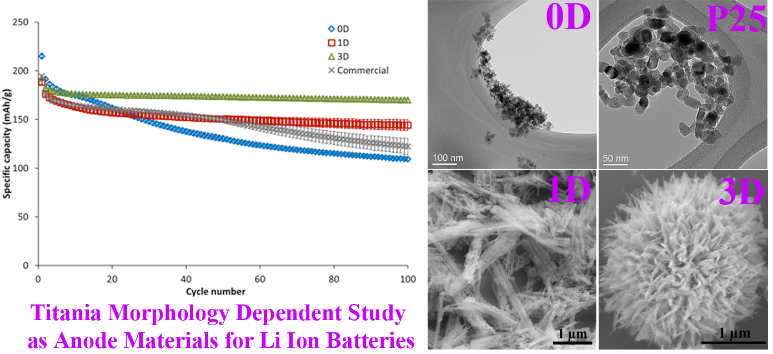
3. Titanium oxide is a ubiquitous and commonly used material in the environment. Herein, we have systematically probed the use of various hydrothermally derived titania (TiO2) architectures including zero-dimensional (0D) nanoparticles, one-dimensional (1D) nanowires, and three-dimensional (3D) urchin-like motifs as anode materials for lithium-ion batteries. The structure and morphology of these nanomaterials were characterized using electron microscopy. The surface areas of these materials were quantitatively analyzed through Brunauer–Emmett–Teller(BET) adsorption measurements and were found to be relatively similar for both 1D and 3D samples with a slightly higher surface area associated with the 0D nanoparticles. Hence, to normalize for the surface area effect, readily available 0D commercial nanoparticles (Degussa P25), which possessed a similar surface area to that of as-prepared 1D and 3D materials, were also analyzed. Electrochemical analysis revealed a superior performance of hydrothermally derived 3D urchin-like motifs as compared with both as-prepared 0D and 1D samples as well as commercial Degussa P25. Our studies suggest the greater overall importance of morphology as opposed to surface area in dictating the efficiency of the Li ion diffusion process. Specifically, the 3D urchins yielded consistent rate capabilities, delivering 214, 167, 120, 99, and 52 mAh/g under corresponding discharge rates of 0.1, 1, 10, 20, and 50 C, respectively. Moreover, these 3D motifs gave rise to a stable cycling performance, exhibiting a capacity retention of ~90 % in cycles 1-100 under a discharge rate of 1 C. Furthermore, the rate capability and cycling performance of our 3D hierarchical motifs were (i) comparable to those of anatase TiO2/TiO2-(B) hybrid structures even with little if any electrochemically promising B phase herein and (ii) clearlyenhanced as compared with previous results using similar anatase 3D microspheres.
Ref.: ACS Sustainable Chemistry and Engineering, 4(12), 6299–6312 (2016)
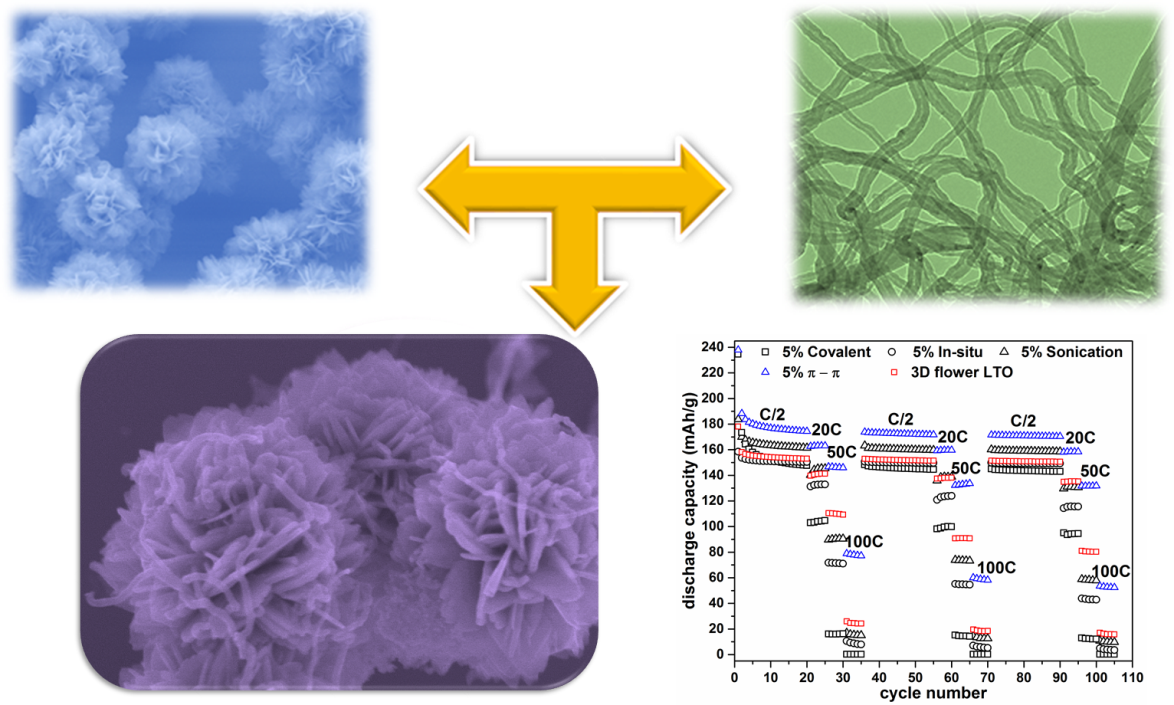
4. Significance of nanoscale attachment modality as an important determinant of observed electrochemical performance. Specifically, controlled loading ratios of MWNTs have been successfully anchored onto the surfaces of a unique “flower-like” Li4Ti5O12 (LTO) micro-scale sphere motif, for the first time, using a number of different and distinctive preparative approaches, including (i) a sonication method, (ii) an in situ direct-deposition approach, (iii) a covalent attachment protocol, as well as (iv) a π-π interaction strategy. In terms of structural characterization, the composites generated by physical sonication as well as non-covalent π-π interactions retained the intrinsic hierarchical “flower-like” morphology and exhibited a similar crystallinity profile as compared with that of pure LTO. By comparison, the composite prepared by an in situ direct deposition approach yielded not only a fragmented LTO structure, likely due to the possible interfering presence of the MWNTs themselves during the relevant hydrothermal reaction, but also a larger crystallite size, owing to the higher annealing temperature associated with its preparation. Finally, the composite created via covalent attachment was covered with an amorphous insulating linker, which probably led to a decreased contact area between the LTO and the MWNTs and hence, a lower crystallinity in the resulting composite. Electrode tests suggested that the composite generated by π-π interactions out-performed the other three analogous heterostructures, due to a smaller charge transfer resistance as well as a faster Li-ion diffusion. In particular, the LTO-MWNT composite, produced by π-π interactions, exhibited a reproducibly high rate capability as well as a reliably solid cycling stability, delivering 132 mA h g-1 at 50 C, after 100 discharge/charge cycles, including 40 cycles at a high (>20 C) rate. Such data denote the highest electrochemical performance measured to date as compared with any LTO-carbon nanotube-based composite materials previously reported, under high discharge rate conditions, and tangibly underscore the correlation between preparative methodology and the resulting performance metrics.
Ref.:J. Electrochem. Soc., v.164, A524 (2017).
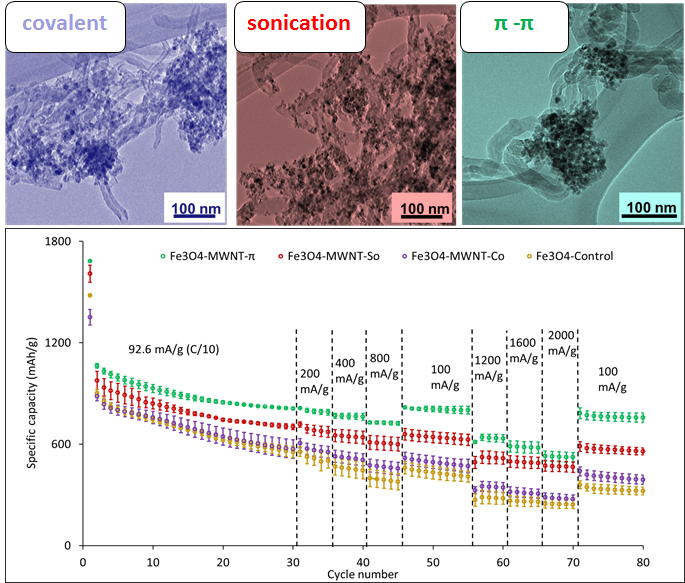
5. Fe3O4 nanoparticles (NPs) with an average size of 8-10 nm and a loading ratio of 50 wt% have been successfully attached onto the external surfaces of MWNTs by means of three different preparative approaches, namely a sonication method, a covalent attachment protocol, as well as a non-covalent π-π interaction strategy. Specifically, the Fe3O4 NPs associated with the sonication method lie directly on the outer surfaces of the MWNTs. Particles covalently attached onto the MWNTs formed amide chemical bonds through the mediation of the amorphous (3-aminopropyl) triethoxysilane (APTES) linker. Finally, particles were anchored non-covalently onto the underlying conjugated MWNTs via an aromatic 4-mercaptobenzoic acid (4-MBA) linker. Both structural and electrochemical characterization protocols have been used to correlate the electrode performance with the corresponding attachment strategies. Fe3O4-MWNT composites generated by the π-π interaction strategy delivered 813, 768, 729, 796, 630, 580, 522, and 762 mAh/g under rates of 200, 400, 800, 100, 1200, 1600, 2000, and 100 mA/g, with 72% retention between cycles 2 and 80, demonstrating both higher capacity and better cycling stability as compared with analogues derived from the physical sonication as well as covalent attachment strategies. This finding may be attributed to the enhanced charge and ion transport coupled with retention of physical contact with the underlying MWNTs after a large volume change during cycling. Our collective data suggest that the non-covalent π-π attachment modality is a more effective preparative strategy for enhancing the performance of MWNT-Fe3O4composite electrodes after full discharge.
Ref.:ECSJournal of Solid State Science and Technology, v.6, M3122 (2017).
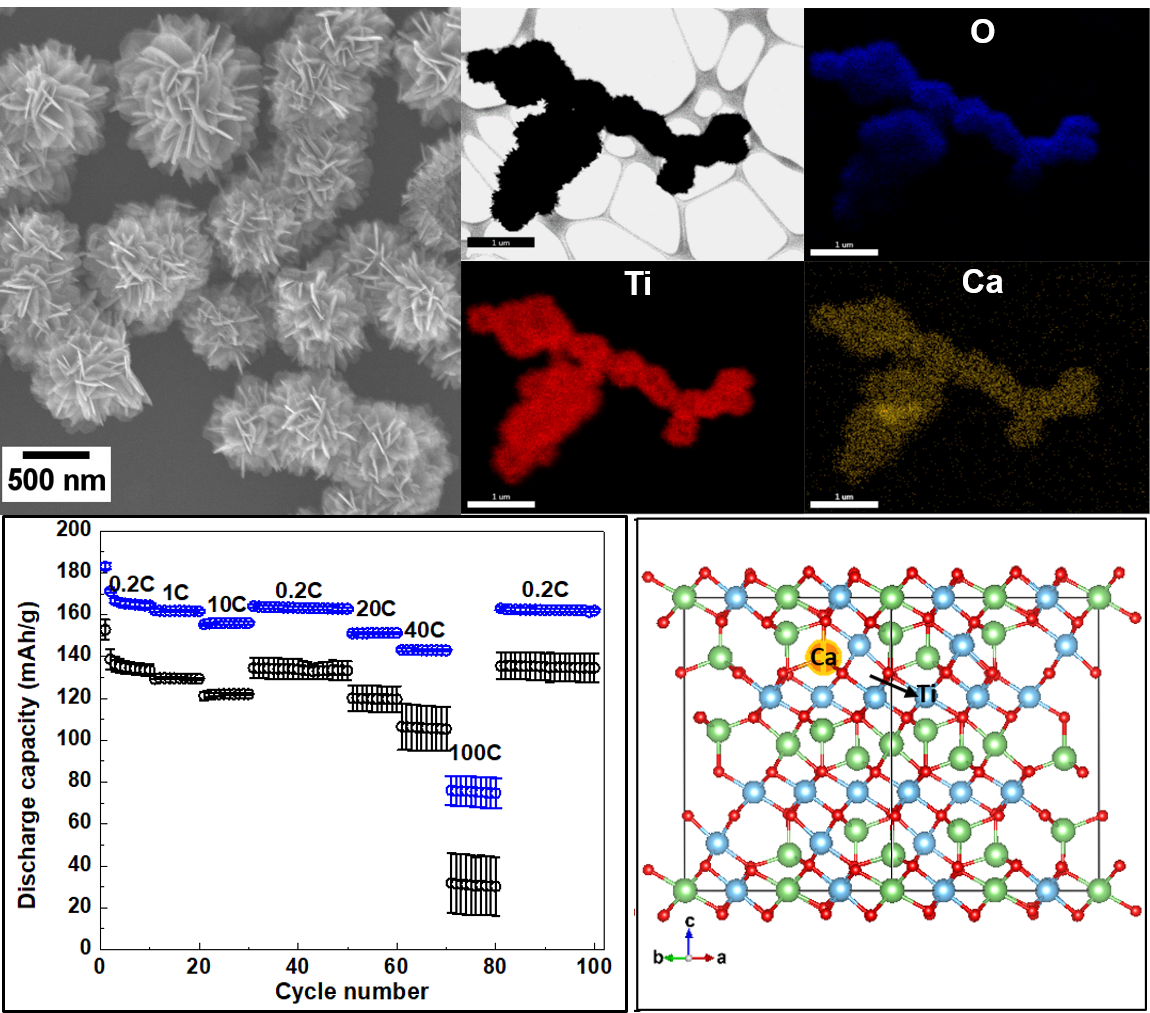
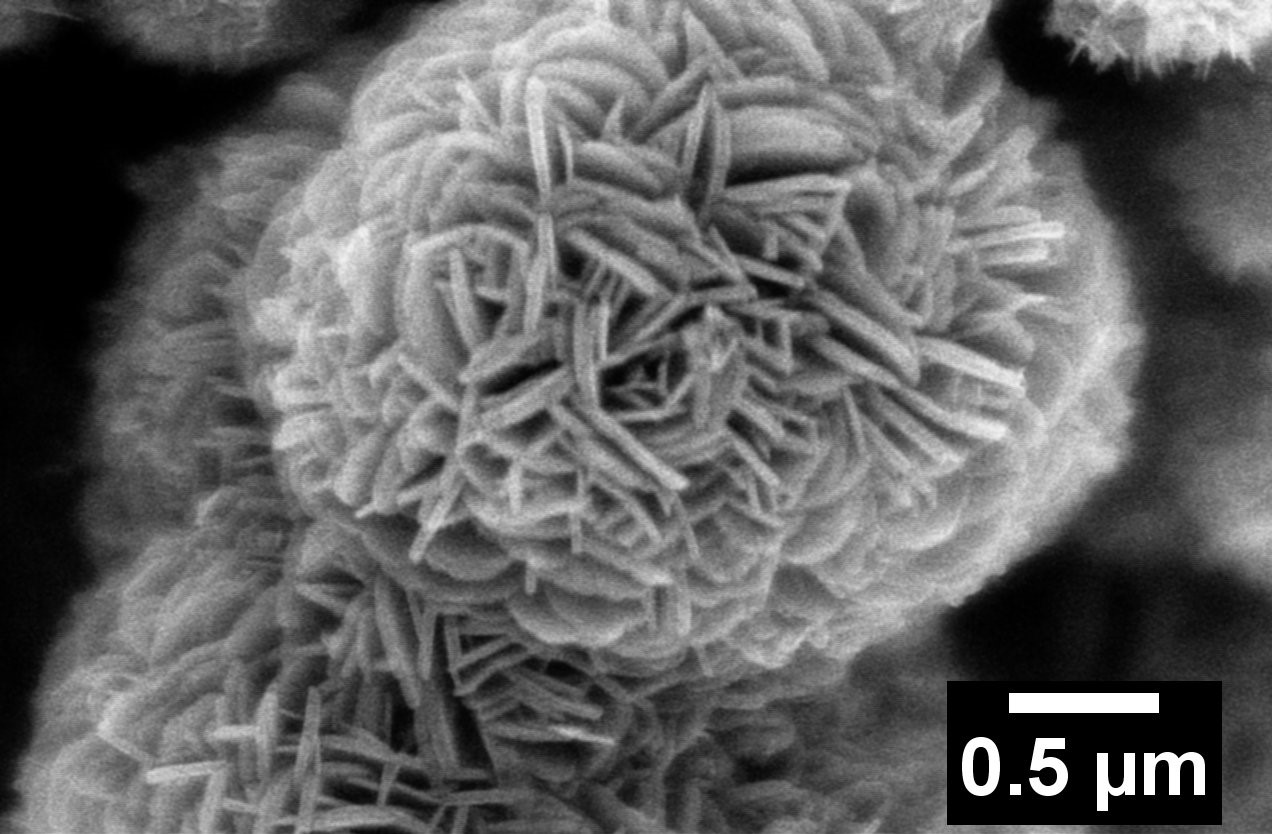
6. Doped motifs offer an intriguing structural pathway towards improving conductivity for battery applications. Specifically, Ca-doped, three-dimensional ‘flower-like’ Li4-xCaxTi5O12 (‘x’ = 0, 0.1, 0.15, and 0.2) micron-scale spheres have been successfully prepared for the first time using a simple and reproducible hydrothermal reaction followed by a short calcination process. The products were experimentally characterized by means of X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) mapping, inductively coupled plasma optical emission spectrometry (ICP-OES), X-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge-discharge testing. Calcium dopant ions were shown to be uniformly distributed within the LTO structure without altering the underlying ‘flower-like’ morphology. The largest lattice expansion and the highest Ti3+ratios were noted with XRD and XPS, respectively, while increased charge transfer conductivity and decreased Li+ion diffusion coefficients were displayed in EIS for the Li4-xCaxTi5O12 (‘x’ = 0.2) sample. The ‘x’ = 0.2 sample displayed a higher rate capability, an excellent reversibility, and a superior cycling stability, delivering 151 and 143 mAh/g under discharge rates of 20C and 40C at cycles 60 and 70, respectively. In addition, a high cycling stability was demonstrated with a capacity retention of 92% after 300 cycles at a very high discharge rate of 20C. In addition, first principles’ calculations based on density functional theory (DFT) were conducted with the goal of further elucidating and understanding the nature of the doping mechanism in this study. The DFT calculations not only determined the structure of the Ca-doped Li4Ti5O12, which was found to be in accordance with the experimentally measured XPD pattern, but also yielded valuable insights into the doping-induced effect on both the atomic and electronic structures of Li4Ti5O12.
Ref.: Chem. Mater., 30(3), 671–684 (2018).
7. We report on the synthesis of submicron Li1+xV3O8 fibers through a facile mixed ethanol/water solution-mediated solvothermal route in the absence of surfactants. All the raw materials used are commercially available, relatively inexpensive, and low-toxic, and can be handled in an ambient atmosphere, rendering this synthetic route as reasonably facile and efficient. To ensure sample crystallinity and purity, we introduced a post-annealing treatment at 500°C. The monoclinic phase formation of the fiber sample was probed in detail using a series of X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), infrared spectroscopy (IR), X-ray photoelectron spectroscopy (XPS), high resolution transmission electron microscopy (HR-TEM) and selected area electron diffraction (SAED) measurements. Both morphology and chemical composition could be carefully and systematically tuned in terms of generating a class of novel, pure, and well-defined motifs of Li1+xV3O8. A plausible mechanism for the formation of submicron-diameter fibers has been discussed in addition to the expected phase transformation within our Li-V-O materials. Our comprehensive study should provide for needed fundamental insights into putting forth a viable synthesis strategy for the generation of well-defined morphological variants of layered oxide materials for battery applications.
Ref.: Crystal Growth and Design, 18(4), 2055–2066 (2018).
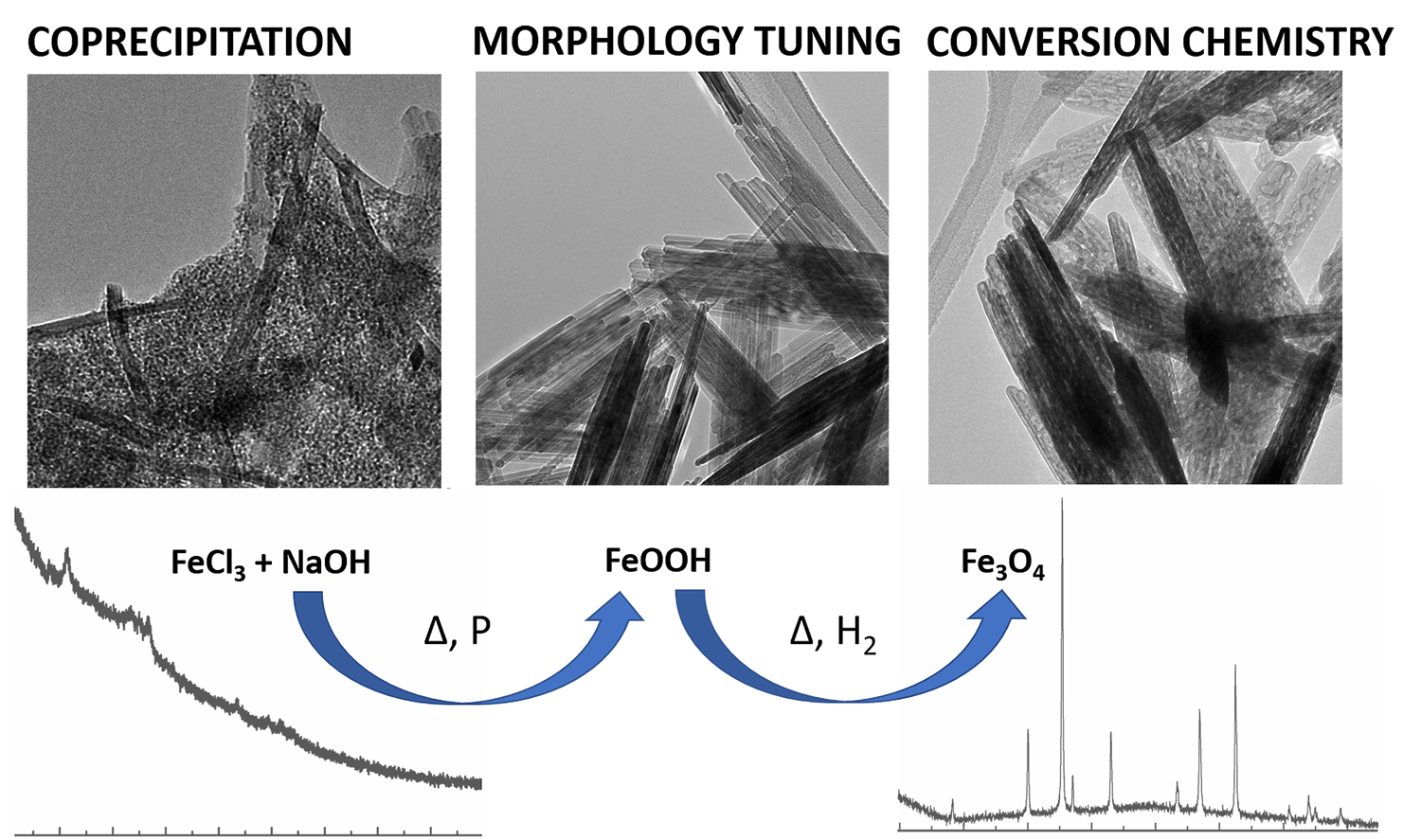
8. As a matter of synthetic novelty, Fe3O4 (magnetite) nanorods (NRs) have been successfully generated using a reproducible four-step protocol, wherein goethite is initially produced, morphologically tuned, chemically treated with a passivating agent to reduce aggregation, and ultimately converted to magnetite by thermal annealing within a reductive atmosphere. Our equally important objective was in correlating electrochemical behavior with the unique morphology of these Fe3O4 anode materials. As such, both conventionally coated and binder-free electrodes were tested using as-prepared magnetite NRs and nanoparticles (NPs) with controlled crystallite size as the active materials. Our study revealed that both the NR and NP Fe3O4 materials were amenable to effective binder-free electrode design. For the conventionally coated electrodes, the NR electrodes demonstrated an improved rate capability using a sequential discharge/charge current density profile as compared with that for corresponding NP electrodes. Most significantly, within the cycling stability test, the NR electrode delivered a high and stable capacity with a superior capacity retention relative to that of the NP for more than 50 cycles in half cells and 100 cycles in full cells. These data in particular showcase the undeniable benefits of the anisotropic structure of the material.
Ref.: ACS Applied Energy Materials, v.2, 4801−4812 (2019).
9. Solution-based, anionic doping represents a convenient strategy with which to improve upon the conductivity of candidate anode materials such as Li4Ti5O12 (LTO). As such, herein, we have primarily devised novel synthetic hydrothermally-inspired protocols, aimed at the large scale production of unique halogen-doped, micron-scale, three-dimensional, hierarchical LTO flower-like motifs. Though we have explored F doping, we have concentrated herein primarily on the use of Cl dopants. Several experimental variables, such as dopant amount, lithium hydroxide concentration, and titanium butoxide purity, were probed and perfected. Furthermore, the Cl doping process did not damage the intrinsic LTO morphology. Our analysis, based on interpreting a compilation of SEM, XRD, XPS, and TEM-EDS results, was used to determine an optimized dopant concentration of Cl. Electrochemical tests demonstrated an increased capacity via cycling of 12% for a doped sample as compared with pristine LTO. Moreover, the Cl-doped LTO sample described in this study exhibited the highest discharge capacity yet reported for this material at 120 mAh/g, obtained with an observed rate of 2C. Overall, these data suggest that the Cl dopant likely enhances not only the ion transport capabilities but also the overall electrical conductivity of our as-prepared structures. To help explain these favorable findings, theoretical DFT calculations were used to postulate that the electronic conductivity and Li diffusion were likely improved by partially reducing Ti4+ to Ti3+ and widening the Li migration channel.
Ref.: Special Issue on Low Temperature Solution Route Approaches to Oxide Functional Nanoscale Materials, invited contribution, Chem. Eur. J., 26(42), 9389 – 9402 (2020).
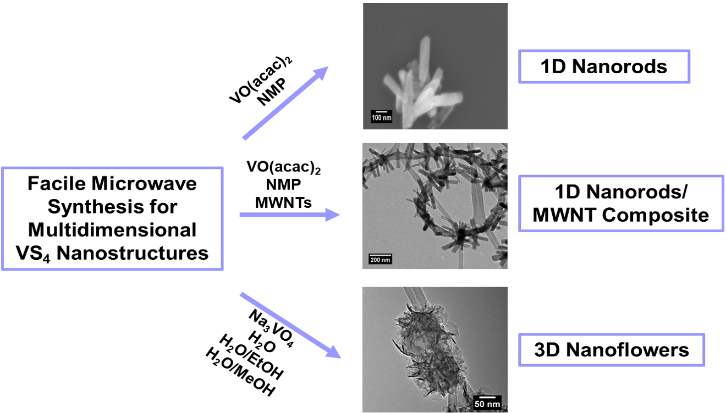
10. A novel facile, fast, and efficient microwave-assisted method was developed to synthesize a number of diverse nanostructured motifs (ranging from nanorods to nanoflowers) of VS4 along with its associated composite heterostructures, VS4/MWNT (i.e., multi-walled carbon nanotubes). In particular, we have probed and correlated the effects of a number of specific experimental variables, including primarily precursor, solvent, temperature, and time. We noted that nanorods formed more readily with VO(acac)2 as the vanadium precursor and n-methyl-2-pyrrolidone (NMP) as a polar reaction solvent. By contrast, we determined that hierarchical three-dimensional (3D) nanoflower-like assemblies, ranging in size from 100 to 200 nm in average diameter, could be controllably synthesized by using Na3VO4 as the vanadium precursor and an aqueous water: polar solvent mixture as the reaction medium. We also observed that VS4 disintegrates, when in the presence of either air, solution, or a combination of these environments, and established that the extent of VS4 nanorod decomposition could be almost fully prevented by storage under nitrogen. From an applications’ perspective, our VS4 is electrochemically active, and shows behavior, consistent with the literature. In particular, as compared with pristine VS4 nanorods alone, we observed enhanced electrochemical activity with (i) 3D hierarchical flower-like motifs, (ii) unique necklace-like VS4 nanorod - MWNT composites, and (iii) samples in which VS4 nanorods had been previously annealed. Moreover, we found that the rational application of specific physical and chemical processing treatments, such as (i) thermal annealing to improve crystallinity, (ii) the addition of MWNTs to form conductive composites, and (iii) the evolution of morphology from one-dimensional (1D) nanorods to more complex 3D nanoflowers were favorable to the resulting electrochemical performance with respect to increasing stability and reversibility.
Ref.:ACS Sustainable Chemistry and Engineering, 8(44), 16397-16412 (2020).
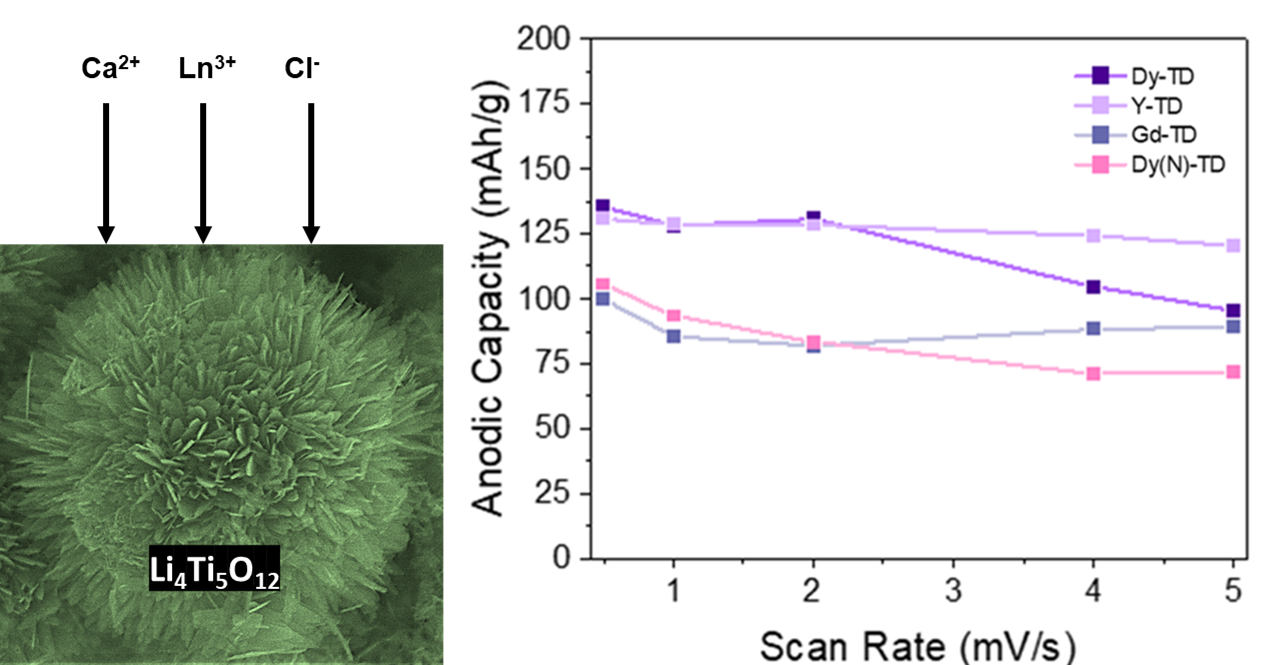
11. This study thoroughly investigated the synthesis of not only 4 triply-doped metal oxides but also 5 singly-doped analogues of Li4Ti5O12for electrochemical applications. In terms of synthetic novelty, the triply-doped materials were fabricated using a relatively facile hydrothermal method for the first-time, involving the simultaneous substitution of Ca for the Li site, Ln (i.e., Dy, Y, or Gd) for the Ti site, and Cl for the O site, respectively. Based on XRD, SEM, and HRTEM-EDS measurements, the resulting materials, incorporating a relatively homogeneous and uniform dispersion of both the single and triple dopants, exhibited a micron-scale flower-like morphology, that remained apparently undamaged by the doping process. Crucially, the surface chemistry of all of the samples was probed using XPS so to analyze any nuanced changes, associated with either different lanthanide dopants or the identity of the metal precursor types involved. In the latter case, it was observed that the use of a nitrate salt precursor versus that of a chloride salt enabled not only a higher lanthanide incorporation but also the potential for favorable N-doping, all of which promoted a concomitant increase in conductivity, due to the increase in Ti3+ content. In terms of the choice of lanthanide system, it was observed via CV analysis that dopant incorporation generally (albeit with some notable exceptions, especially with Y-based materials) led to the formation of higher amounts of Ti3+ species within both the singly and triply-doped materials, which consequentially led to the potential for increased diffusivity and higher mobility of Li+ species with the possibility for enabling greater capacity within these classes of metal oxides.
Ref.: invited contribution, ACS Physical Chemistry Au, 2(4), 331-345 (2022).
12. Using a variety of synthetic protocols including hydrothermal and microwave-assisted methods, the morphology of as-prepared magnetite has been reliably altered as a means of probing the effect of facet variations upon the resulting electrochemical processes measured. In particular, motifs of magnetite, measuring ~100 to 200 nm in diameter, were variously prepared in the form of cubes, spheres, octahedra, and plates, thereby affording the opportunity to preferentially expose either (111), (220), or (100) planes, depending on the geometry in question. We deliberately prepared these samples, characterized using XRD and SEM, in the absence of a carbonaceous surfactant to enhance their intrinsic electrochemical function. Herein, we present a direct electrochemical comparison of specifically modified shape morphologies possessing 3 different facets and their impact as electrode materials for Li-ion batteries. Our overall data suggest that the shapes exhibiting the largest deliverable capacities at various current densities incorporated the highest surface energy facets, such as exposed (220) planes in this study. The faceted nature of different morphologies highlighted a trend in electrochemistry of (220) > (111) > (100); moreover, the degree of aggregation and polydispersity in prepared samples were found to play key roles as well.
Ref.:Journal of The Electrochemical Society, 169(8), 080512 / 1-9 (2022)
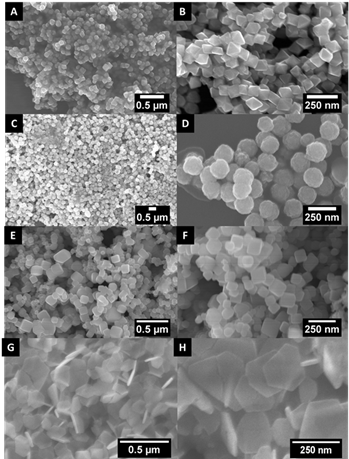
13.
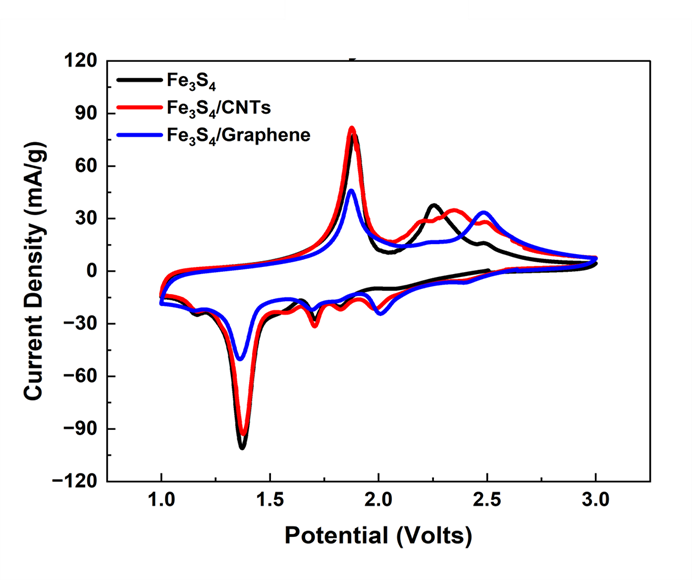
The syntheses of FeS2 and Fe3S4 nanomaterials were optimized using a novel facile, surfactant-free, and microwave-assisted, one-pot synthesis method, run under ambient and reasonably mild reaction conditions. Synthetic parameters, such as metal precursor salt identity, reaction time, reaction temperature, metal: sulfur molar ratios, and solvent combinations, were all systematically investigated and optimized. A series of FeS2 (pyrite) samples was initially fabricated using thioacetamide as the sulfur precursor to generate a distinctive, uniform octahedra-based morphology. Switching the sulfur precursor from thioacetamide to L-cysteine resulted in a corresponding transformation in not only chemical composition from FeS2 to an iron thiospinel structure, Fe3S4 (otherwise known as greigite), but also an associated morphological evolution from octahedra to nanosheet aggregates. The study of these materials has enabled crucial insights into the formation mechanisms of these materials under a relatively non-conventional microwave-assisted setting. Furthermore, in separate experiments, multi-walled carbon nanotubes (MWNTs) and graphene were added in with underlying metal sulfide species to create conductive Fe-S / MWNT composites and Fe-S / graphene composites, respectively. The method of addition of either MWNTs or graphene was also explored, wherein an ‘ex-situ’ synthetic procedure was found to be the least disruptive means of attachment and immobilization onto iron sulfide co-reagents as a means of preserving the latter’s inherent composition and morphology. The redox acidity for the parent material and associated composites demonstrates the utility of our as-developed synthetic methods for creating motifs relevant for electrochemical applications, such as energy storage.
Ref.: invited contribution, Journal of Physics: Materials, 6(2), 024005 / 1-16 (2023).