Nanotube-Nanocrystal Heterostructures
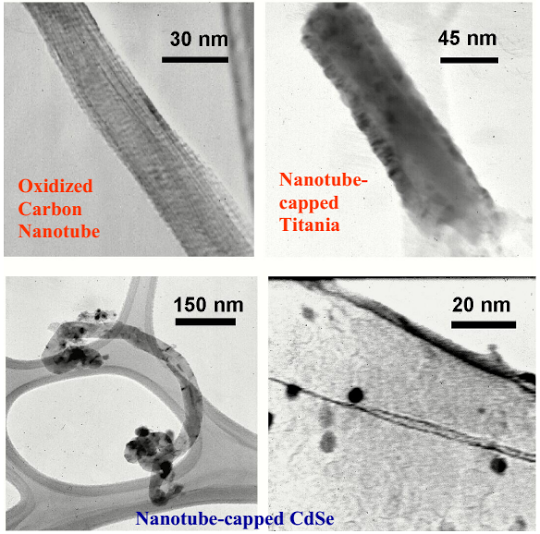
1. Linker-mediated, wet-chemical synthesis of molecular heterostructures consisting of carbon nanotubes and semiconductor nanocrystals (CdSe and TiO2) with demonstration of charge transfer.
Ref.: Nano Letters, v.2, 195 (2002).
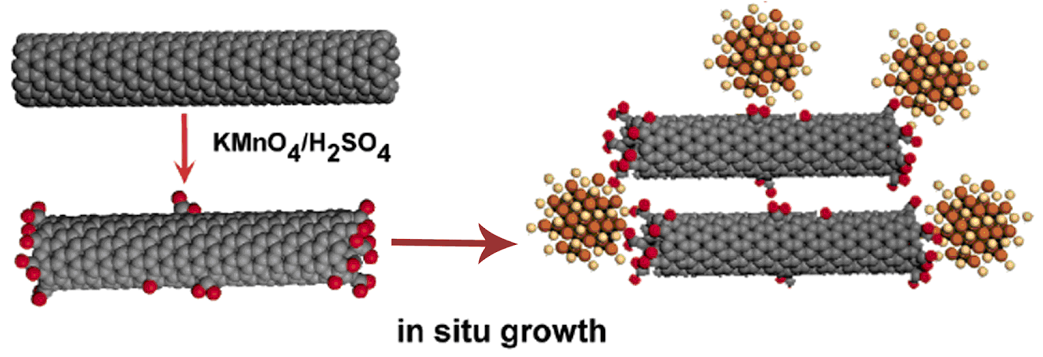
2. Synthesis of a seamless nanotube-nanocrystal junction interface by using carbon nanotubes as ‘templates’ for subsequent nanocrystal formation.In situ nucleation and controlled growth of crystalline CdTe and CdSe quantum dots on the surfaces of oxidized (i.e. acid-treated or ozonized) multi-walled and single-walled carbon nanotubes. Specifically, we coordinate metallic precursors of quantum dots directly onto the surfaces of processed, ‘oxygenated’ nanotubes and then, proceed with in situ growth. The resulting network of molecular-scale ‘fused’ nanotube-nanocrystal heterojunctions demonstrates a controlled synthetic route to the synthesis of complex nanoscale heterostructures. The work demonstrates the creation of a sharp junction interface, whose properties are manipulable and hence, predictable. The implication is that the direction of charge and electron transfer can be modulated. This is of importance to the development of photoelectrochromic materials, in particular optical storage devices.
Refs.: Chem. Commun., 1866 (2004); Adv. Mater. (inside cover), v.16, 34 (2004); and J. Am. Chem. Soc., v.125, 10342 (2003);
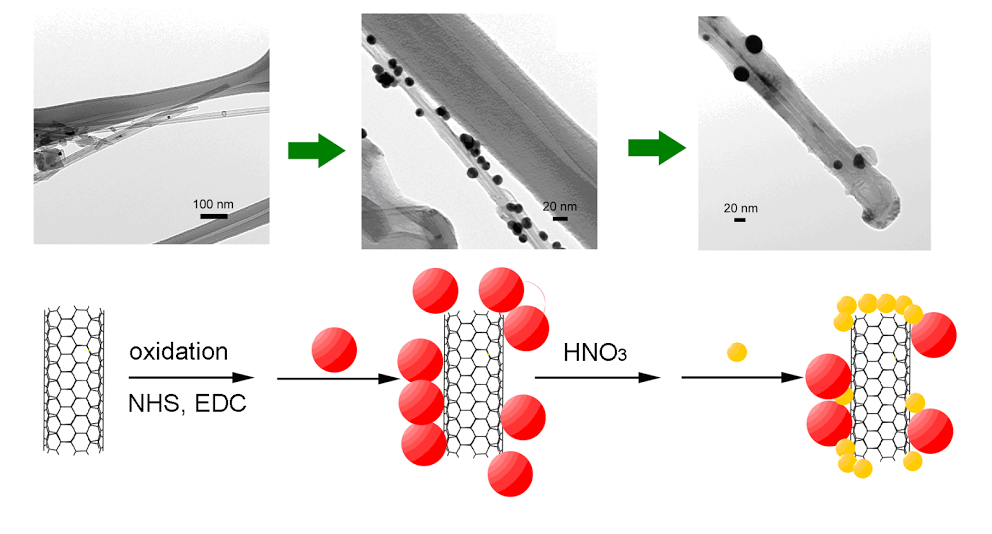
3. We have demonstrated a covalent route towards site-selective synthesis of multi-walled carbon nanotube (MWNT)-nanoparticle conjugates containing two different types of nanoscale species, i.e. Au nanoparticles and CdSe QDs. We have systematically and quantitatively probed the effects of varying oxidation treatments, precursor concentrations, and incubation times in order to rationally affect the spatial coverage and distribution of either Au NPs or semiconducting QDs on the MWNT sidewalls and tips. The degree of nanoparticulate coverage was found to primarily vary with the intensity of the oxidation treatment, though the hydrophobicity of the nanotube as well as the chemical and steric characteristics of the nanocrystals also played a role in determining the ultimate architecture. In general, the stronger the oxidation treatment, the denser the coating of nanoparticles and/or quantum dots on the nanotube surface. In addition, the use of larger concentrations of precursor nanocrystals along with longer incubation times was also conducive to the observation of higher nanoparticle densities on our nanotube templates. Interesting charge-transfer, electromagnetic enhancement, as well as energy transfer behavior between CNTs and the attached nanoparticles/quantum dots have been observed, and will likely render such conjugates as key components in a range of nanoscale devices important for photocatalytic and solar applications.
Ref.: Chem. Mater., v.21, 682 (2009).
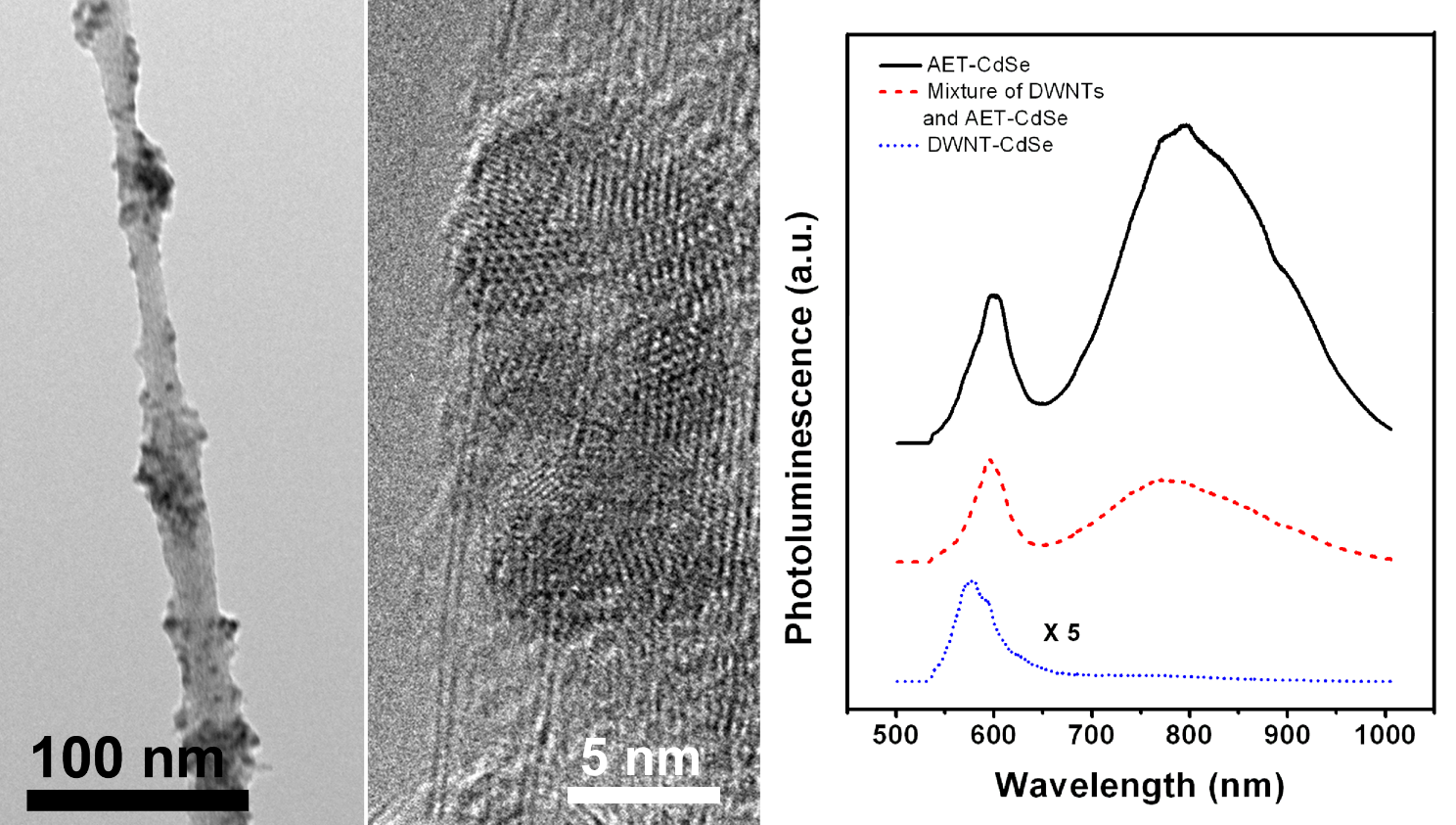
4. The unique electronic structure and optical properties of double-walled carbon nanotubes (DWNTs) have rendered them as a key focus material of research in recent years. However, the incorporation of DWNTs with quantum dots (QDs) into nanocomposites via a covalent chemical approach as well as the optical properties of the composites has rarely been explored. In particular, we have been interested in this model system as to whether nanomaterial heterostructures can provide efficient pathways for charge separation relative to loss mechanisms such as recombination. In this specific work, the synthesis of DWNT-CdSe quantum dot (QD) heterostructures using a conventional covalent protocol has been demonstrated. CdSe QDs with terminal amino groups have been conjugated onto the surfaces of oxidized DWNTs by the formation of amide bonds. The observed trap emission of CdSe is thought to arise from the presence of 2-aminoethanethiol capping ligands, and is effectively quenched upon conjugation with the DWNT surface due to the charge transfer from CdSe to DWNTs.
In further experiments, DWNT-CdSe heterostructures with the individual nanoscale building blocks linked together by 4-aminothiophenol (4-ATP) have been successfully synthesized using two different and complementary routes, i.e. covalent attachment and π-π stacking. Specifically, using a number of characterization methods, we have probed the effects of these differential synthetic coupling approaches on the resulting CdSe quantum dot (QD) coverage on the underlying nanotube template as well as the degree of charge transfer between the CdSe QDs and the DWNTs. In general, based on microscopy and spectroscopy data collectively, we noted that heterostructures generated by non-covalent π-π stacking interactions evinced not only higher QD coverage density but also possibly more efficient charge transfer behavior as compared with their counterparts produced using covalent linker-mediated protocols.
Refs.: Dalton Transactions, v.43(20), 7480 (2014) and J. Phys. Chem. C, v.114, 8766 (2010).
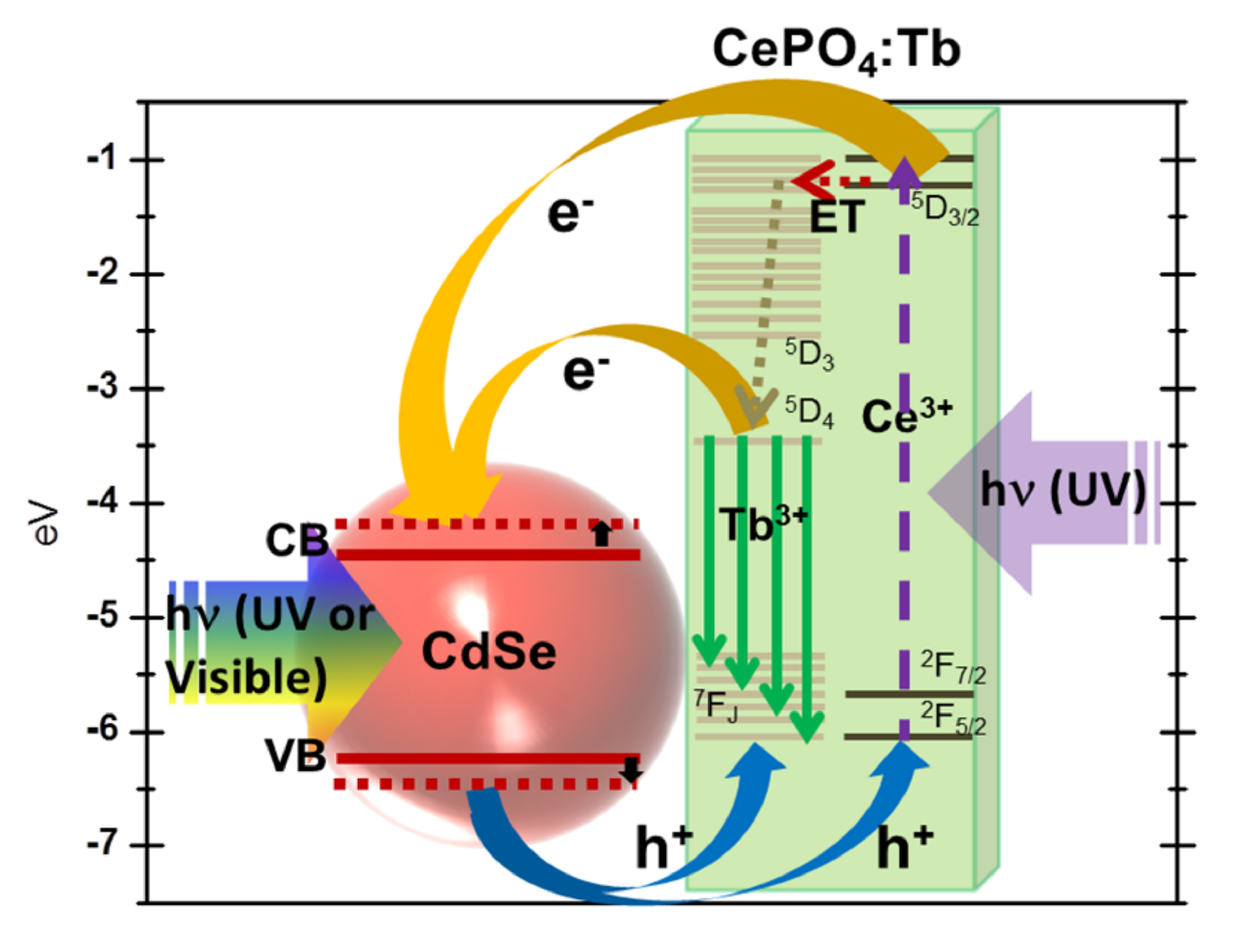
5. We report on the synthesis, structural characterization, and intrinsic charge transfer processes associated with novel luminescent zero-dimensional (0-D) CdSe nanocrystal – one-dimensional (1-D) CePO4: Tb nanowire composite heterostructures. Specifically, ~4 nm CdSe quantum dots (QDs) have been successfully anchored onto high-aspect ratio CePO4: Tb nanowires, measuring ~65 nm in diameter and ~2 μm in length. Composite formation was confirmed by high resolution transmission microscopy, energy-dispersive X-ray spectroscopy mapping, and confocal microscopy. Photoluminescence (PL) spectra, emission decay, and optical absorption of these nanoscale heterostructures were collected and compared with those of single, discrete CdSe QDs and CePO4: Tb nanowires. We show that our composite heterostructure evinces both PL quenching and a shorter average lifetime as compared with unbound CdSe QDs and CePO4: Tb nanowires. We propose that a photo-induced 0D-1D charge transfer process occurs between CdSe and CePO4: Tb and that it represents the predominant mechanism, accounting for the observed PL quenching and shorter lifetimes noted in our composite heterostructures. Data are additionally explained in the context of the inherent energy level alignments of both CdSe QDs and CePO4: Tb nanowires.
Ref.: J. Phys. Chem. C, v.118, 5671 (2014).
Ref.: RSC Advances, v.4., 34963-34980 (2014).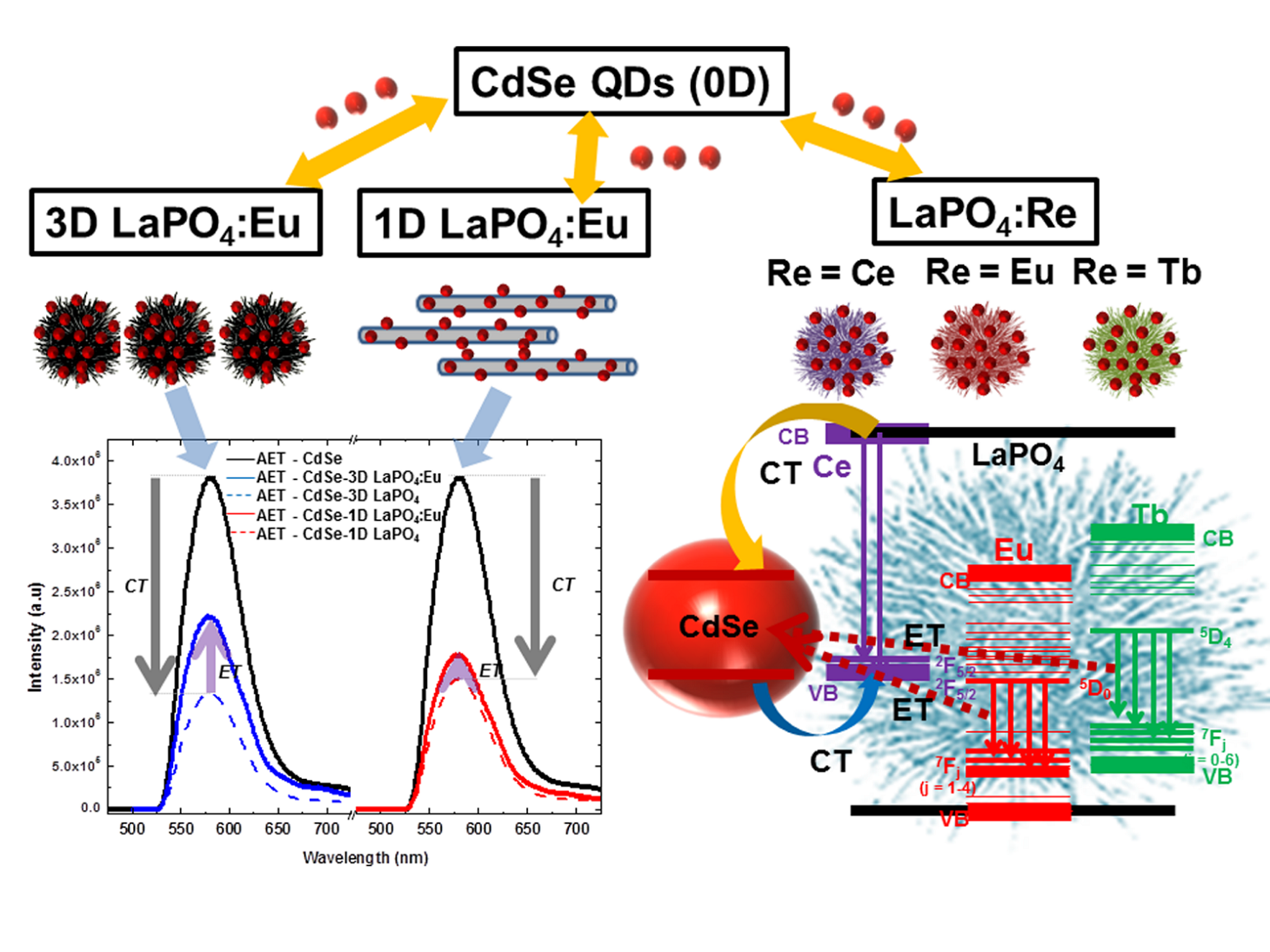
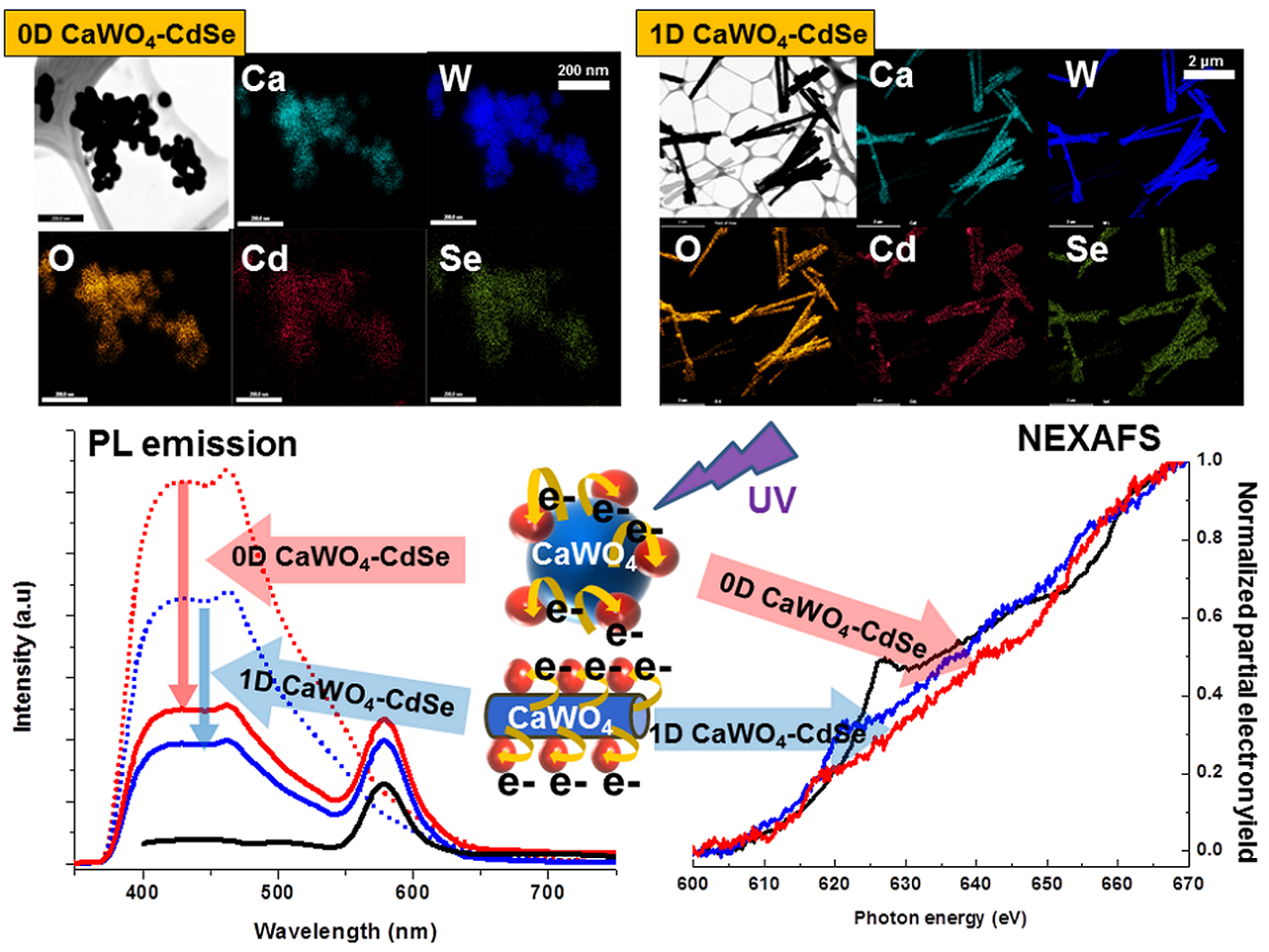
7. In this report, we synthesize and characterize the structural and optical properties of novel heterostructures composed of (i) semiconducting nanocrystalline CdSe quantum dot (QDs) coupled with (ii) both one and zero-dimensional (1D and 0D) motifs of self-activated luminescence CaWO4 metal oxides. Specifically, ~4 nm CdSe QDs have been anchored onto (i) high-aspect ratio 1D nanowires, measuring ~230 nm in diameter and ~3 μm in length, as well as onto (ii) crystalline 0D nanoparticles (possessing an average diameter of ~ 80 nm) of CaWO4 through the mediation of 3-mercaptopropionic acid (MPA) as a connecting linker. Composite formation was confirmed by complementary electron microscopy and spectroscopy (i.e. IR and Raman) data. In terms of luminescent properties, our results show that our 1D and 0D heterostructures evince photoluminescence (PL) quenching and shortened PL lifetimes of CaWO4 as compared with unbound CaWO4. We propose that a photo-induced electron transfer process occurs from CaWO4 to CdSe QDs, a scenario which has been confirmed by NEXAFS measurements and which highlights a decrease in the number of unoccupied orbitals in the conduction bands of CdSe QDs. By contrast, the PL signature and lifetimes of MPA-capped CdSe QDs within these heterostructures do not exhibit noticeable changes as compared with unbound MPA-capped CdSe QDs. The striking difference in optical behavior between CaWO4 nanostructures and CdSe QDs within our heterostructures can be correlated with the relative positions of their conduction and valence energy band levels. In addition, the PL quenching behaviors for CaWO4 within the heterostructure configuration were examined by systematically varying (i) the quantities and coverage densities of CdSe QDs as well as (ii) the intrinsic morphology (and by extension, the inherent crystallite size) of CaWO4 itself.
Ref.: Chem. Mater., v.27, 778–792 (2015).
8. As a first step, we have synthesized and optically characterized a systematic series of one-dimensional (1D) single-crystalline Eu3+-activated alkaline-earth metal tungstate/molybdate solid solution composite CaW1-xMoxO4 (0 ≤ ‘x’ ≤ 1) nanowires of controllable chemical composition using a modified template-directed methodology under ambient room-temperature conditions. Extensive characterization of the resulting nanowires has been performed using X-ray diffraction, electron microscopy, and optical spectroscopy. The crystallite size and single crystallinity of as-prepared 1D CaW1-xMoxO4: Eu3+ (0 ≤ ‘x’ ≤ 1) solid solution composite nanowires increase with increasing Mo component (‘x’). We note a clear dependence of luminescence output upon nanowire chemical composition with our 1D CaW1-xMoxO4: Eu3+ (0 ≤ ‘x’ ≤ 1) evincing the highest photoluminescence (PL) output at ‘x’ = 0.8, amongst samples tested. Subsequently, coupled with either zero-dimensional (0D) CdS or CdSe quantum dots (QDs), we successfully synthesized and observed charge transfer processes in 1D CaW1-xMoxO4: Eu3+ (‘x’ = 0.8) – 0D QD composite nanoscale heterostructures. Our results show that CaW1-xMoxO4: Eu3+ (‘x’ = 0.8) nanowires give rise to PL quenching when CdSe QDs and CdS QDs are anchored onto the surfaces of 1D CaWO4 - CaMoO4: Eu3+ nanowires. The observed PL quenching is especially pronounced in CaW1-xMoxO4: Eu3+(‘x’ = 0.8) – 0D CdSe QD heterostructures. Conversely, the PL output and lifetimes of CdSe and CdS QDs within these heterostructures are not noticeably altered as compared with unbound CdSe and CdS QDs. The difference in optical behavior between 1D Eu3+ activated tungstate and molybdate solid solution nanowires and the semiconducting 0D QDs within our heterostructures can be correlated with the relative positions of their conduction and valence energy band levels. We propose that the PL quenching can be attributed to a photo-induced electron transfer process from CaW1-xMoxO4: Eu3+ (‘x’ = 0.8) to both CdSe and CdS QDs, an assertion supported by complementary NEXAFS measurements.
Ref.: J. Phys. Chem. C, v.119, 3826–3842 (2015).

9. As a model system for understanding charge transfer in novel architectural designs for solar cells, double-walled carbon nanotube (DWNT) – CdSe quantum dot (QD) (QDs with average diameters of 2.3 nm, 3.0 nm, and 4.1 nm, respectively) heterostructures have been fabricated. The individual nanoscale building blocks were successfully attached and combined using a hole-trapping thiol linker molecule, i.e. 4-mercaptophenol (MTH), through a facile, non-covalent π-π stacking attachment strategy. Transmission electron microscopy (TEM) confirmed the attachment of QDs onto the external surfaces of the DWNTs. We herein demonstrate a meaningful and unique combination of near-edge X-ray absorption fine structure (NEXAFS) and Raman spectroscopies bolstered by complementary electrical transport measurements in order to elucidate the synergistic interactions between CdSe QDs and DWNTs, which are facilitated by the bridging MTH molecule that can scavenge photo-induced holes and potentially mediate electron redistribution between the conduction bands in CdSe QDs and the C 2p-derived states of the DWNTs. Specifically, we correlated evidence of charge transfer as manifested by (i) changes in the NEXAFS intensities of π* resonance in the C K-edge and Cd M3-edge spectra, (ii) a perceptible outer tube G-band down-shift in frequency in Raman spectra, as well as (iii) alterations in the threshold characteristics present in transport data as a function of CdSe QD deposition onto the DWNT surface. In particular, the separate effects of (i) varying QD sizes and (ii) QD coverage densities on the electron transfer were independently studied.
Ref.: J. Phys. Chem. C, v.119(47), 26327–26338 (2015). 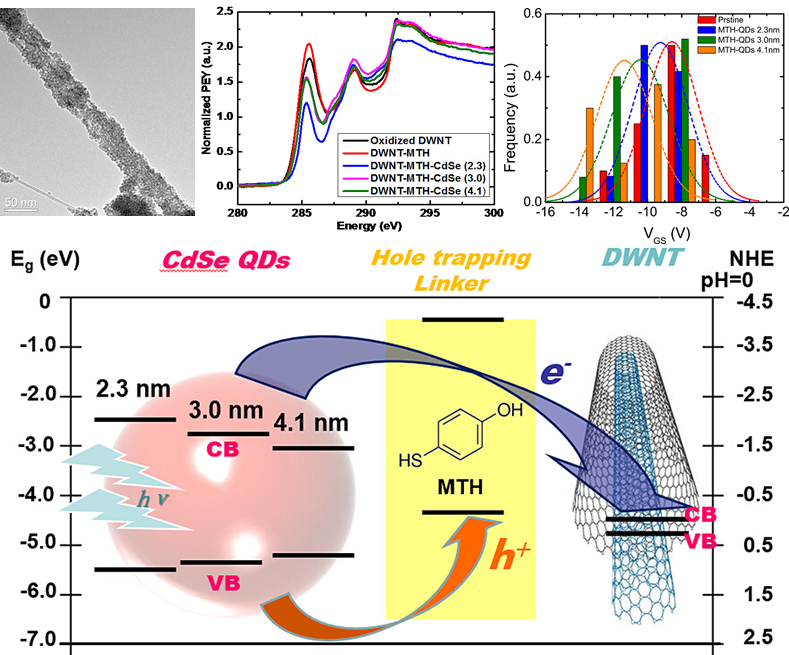
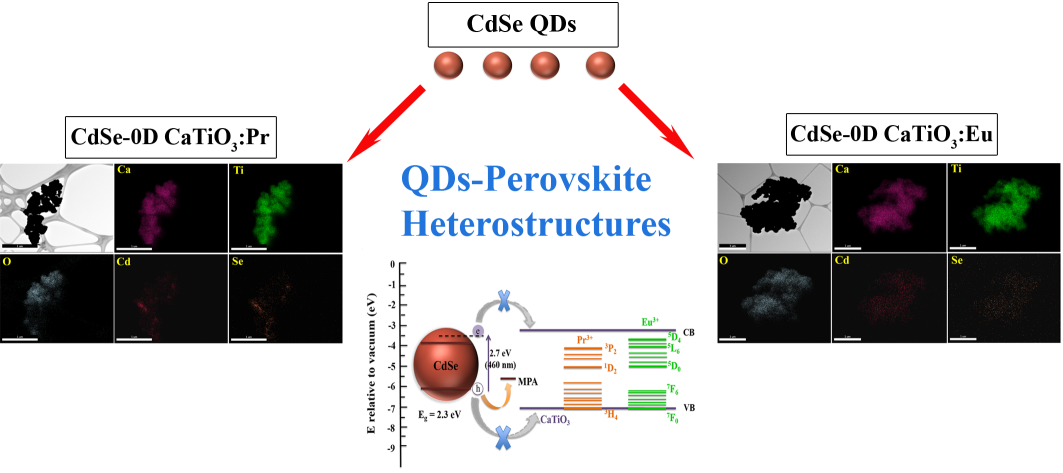
10. We report on the synthesis and structural characterization of novel semiconducting heterostructures composed of cadmium selenide (CdSe) quantum dots (QDs) attached onto the surfaces of novel high-surface area, porous rare-earth-ion doped alkaline earth titanate micron-scale spherical motifs, i.e. both Eu-doped and Pr-doped CaTiO3, composed of constituent, component nanoparticles. These unique metal oxide perovskite building blocks were created by a multi-pronged synthetic strategy involving molten salt and hydrothermal protocols. Subsequently, optical characterization of these heterostructures indicated a clear behavioral dependence of charge transfer in these systems upon a number of parameters such as the nature of the dopant, the reaction temperature, and particle size. Specifically, 2.7 nm diameter ligand-functionalized CdSe QDs were anchored onto sub-micron sized CaTiO3-based spherical assemblies, prepared by hydrothermal methodologies. We found that both the Pr- and Eu-doped CaTiO3displayed pronounced PL emissions, with maximum intensities observed using optimized lanthanide concentrations of 0.2 mol% and 6% mol, respectively. Analogous experiments were performed on Eu doped BaTiO3and SrTiO3 oxide motifs, but CaTiO3 still performed as the most effective host material amongst the three perovskite systems tested. Moreover, the ligand-capped CdSe QD – doped CaTiO3 heterostructures exhibited effective charge transfer between the two individual constituent nanoscale components, an assertion corroborated by the corresponding quenching of their measured PL signals.
Ref.: invited paper, Nanoscale, v.8(4), 2129-2142 (2016).
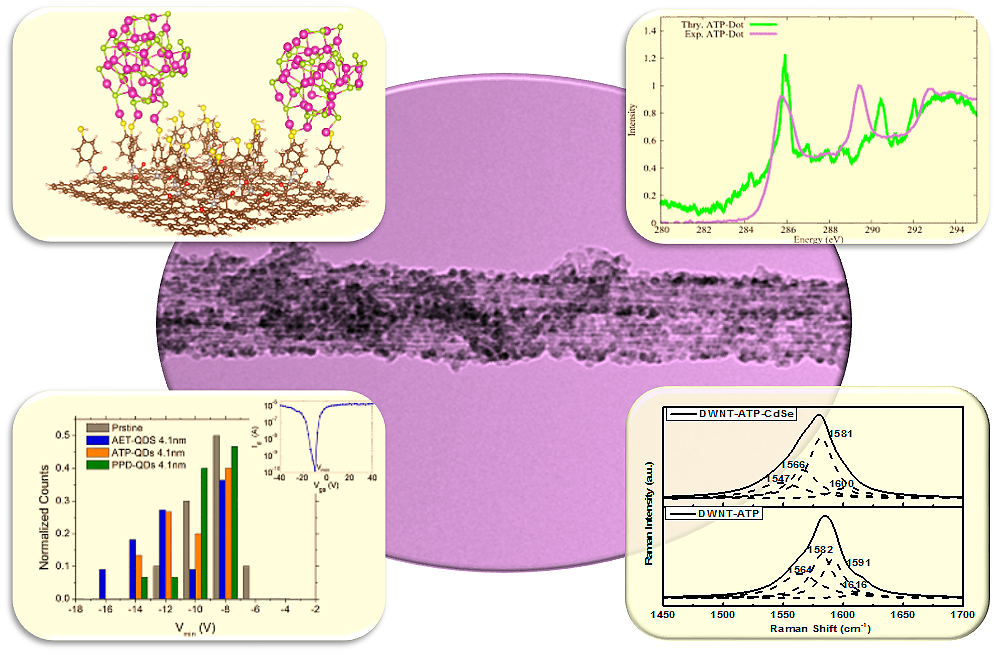
11. As a model system to probe ligand-dependent charge transfer in complex composite heterostructures, we fabricated double-walled carbon nanotube (DWNT) – CdSe quantum dot (QD) composites. Whereas the average diameter of the QDs probed was kept fixed at ~4.1 nm and the nanotubes analyzed were similarly oxidatively processed, by contrast, the ligandsused to mediate the covalent attachment between the QDs and DWNTs were systematically varied to include p-phenylenediamine (PPD), 2-aminoethanethiol (AET), and 4-aminothiophenol (ATP).Herein, we have put forth a unique compilation of complementary data from experiment and theory, including results from transmission electron microscopy (TEM), near-edge X-ray absorption fine structure (NEXAFS) spectroscopy, Raman spectroscopy, electrical transport measurements, and theoretical modeling studies, in order to fundamentally assess the nature of the charge transfer between CdSe QDs and DWNTs, as a function of the structure of various, intervening bridging ligand molecules. Specifically, we correlated evidence of charge transfer as manifested by changes and shifts associated with NEXAFS intensities, Raman peak positions, and threshold voltages both before and after CdSe QD deposition onto the underlying DWNT surface. Importantly, for the first time ever in these types of nanoscale composite systems, we have sought to use theoretical modeling to justify and account for our experimental results. Our overall data suggest that (i) QD coverage density on the DWNTs varies, based upon the different ligand pendant groups used and that (ii) the presence of a p-conjugated carbon framework within the ligands themselves and the electron affinity of the pendant groups collectively play important roles in the resulting charge transfer from QDs to the underlying CNTs.
Ref.: Nanoscale (featured on inside back cover), v.8(34), 15553-15570 (2016).
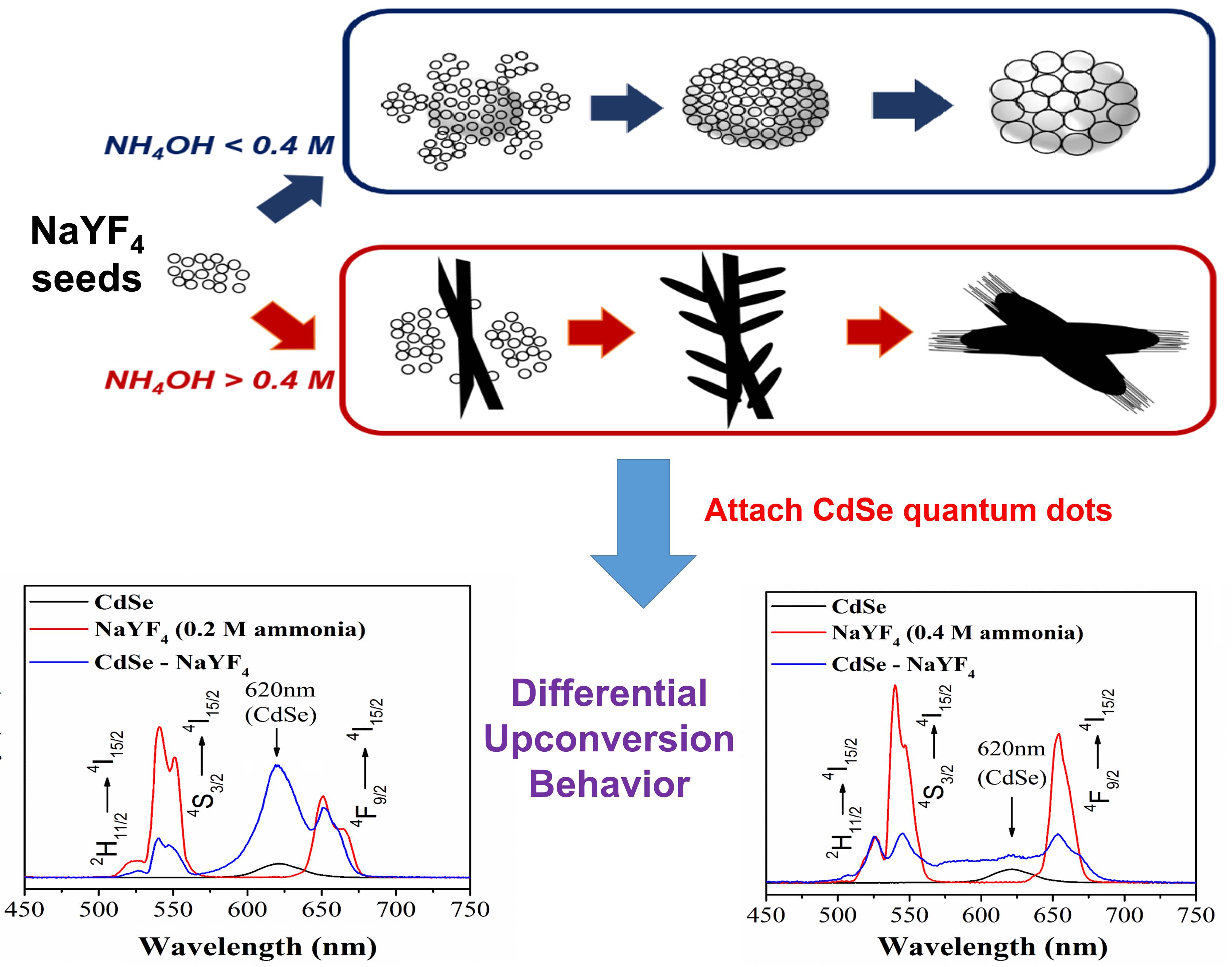
12. Understanding the key parameters necessary for generating uniform Er,Yb co-activated NaYF4 possessing various selected phases (i.e., cubic or hexagonal) represents an important chemical strategy towards tailoring optical behavior in these systems. Herein, we report on a straightforward hydrothermal synthesis in which the separate effects of reaction temperature, reaction time, and precursor stoichiometry in the absence of any surfactant were independently investigated. Interestingly, the presence and concentration of NH4OH appear to be the most critical determinant of phase and morphology. For example, with NH4OH as an additive, we have observed the formation of novel hierarchical nanowire bundles which possess overall lengths of ~5 µm and widths of ~1.5 µm but are composed of constituent component sub-units of long, ultrathin (~5 nm) nanowires. These motifs have yet to be reported as distinctive morphological manifestations of fluoride materials. The optical properties of as-generated structures have also been carefully analyzed. Specifically, we have observed tunable, structure-dependent energy transfer behavior associated with the formation of a unique class of NaYF4-CdSe QD heterostructures, incorporating 0D, 1D, and 3D NaYF4 structures. Our results have demonstrated the key roles of the intrinsic morphology-specific physical surface area and porosity as factors in governing the resulting opto-electronic behavior. Specifically, the trend in energy transfer efficiency correlates well with the corresponding QD loading within these heterostructures, thereby implying that the efficiency of FRET appears to be directly affected by the amount of QDs immobilized onto the external surfaces of the underlying fluoride host materials.
Ref:.Phys. Chem. Chem. Phys.,v.19, 2153 (2017).
13. In this study, we initially created 1D Mn2+-doped ZnS (ZnS: Mn) nanowires (NWs) with a unique optical signature. Specifically, these nanostructures coupled (i) ZnS defect-related self-activated emission spanning from wavelengths of 400 nm to 500 nm with (ii) Mn2+ dopant-induced emission centered at ~580 nm. These doped ZnS nanostructures were initially fabricated for the first time via a template-based co-precipitation approach followed by a post-synthesis annealing process. We subsequently formed novel 1D - 0D heterostructures incorporating ZnS: Mn NWs and AET (2-amino-ethanethiol) - CdSe QDs by assembling annealed ZnS: Mn NWs with AET- capped CdSe QDs as building blocks via a simple technique, involving physical sonication and stirring. Optical analyses of our heterostructures were consistent with charge (hole) and energy transfer-induced quenching of ZnS self-activated emission coupled with hole transfer-related quenching of Mn2+ emission by the QDs. The CdSe QD emission itself was impacted by competing charge (electron) and energy transfer processes occurring between the underlying ZnS host and the immobilized CdSe QDs. Chromaticity analysis revealed the significance of controlling both QD coverage density and Mn2+ dopant ratios in predictably influencing the observed color of our all-inorganic heterostructures. For example, white-light emitting behavior was especially prominent in composites, simultaneously characterized by (i) a 2.22% Mn2+ doping level and (ii) a molar compositional ratio of [ZnS: Mn2+]: [AET-capped CdSe QDs]) of 1: 1.5. Moreover, using these independent chemical ‘knobs’, we have been able to reliably tune for a significant shift within our composites from ‘cold-white’ (9604 K) to ‘warm-white’ (4383 K) light emission.
Ref.:Advanced Optical Materials, v.5, 1700089 (2017).
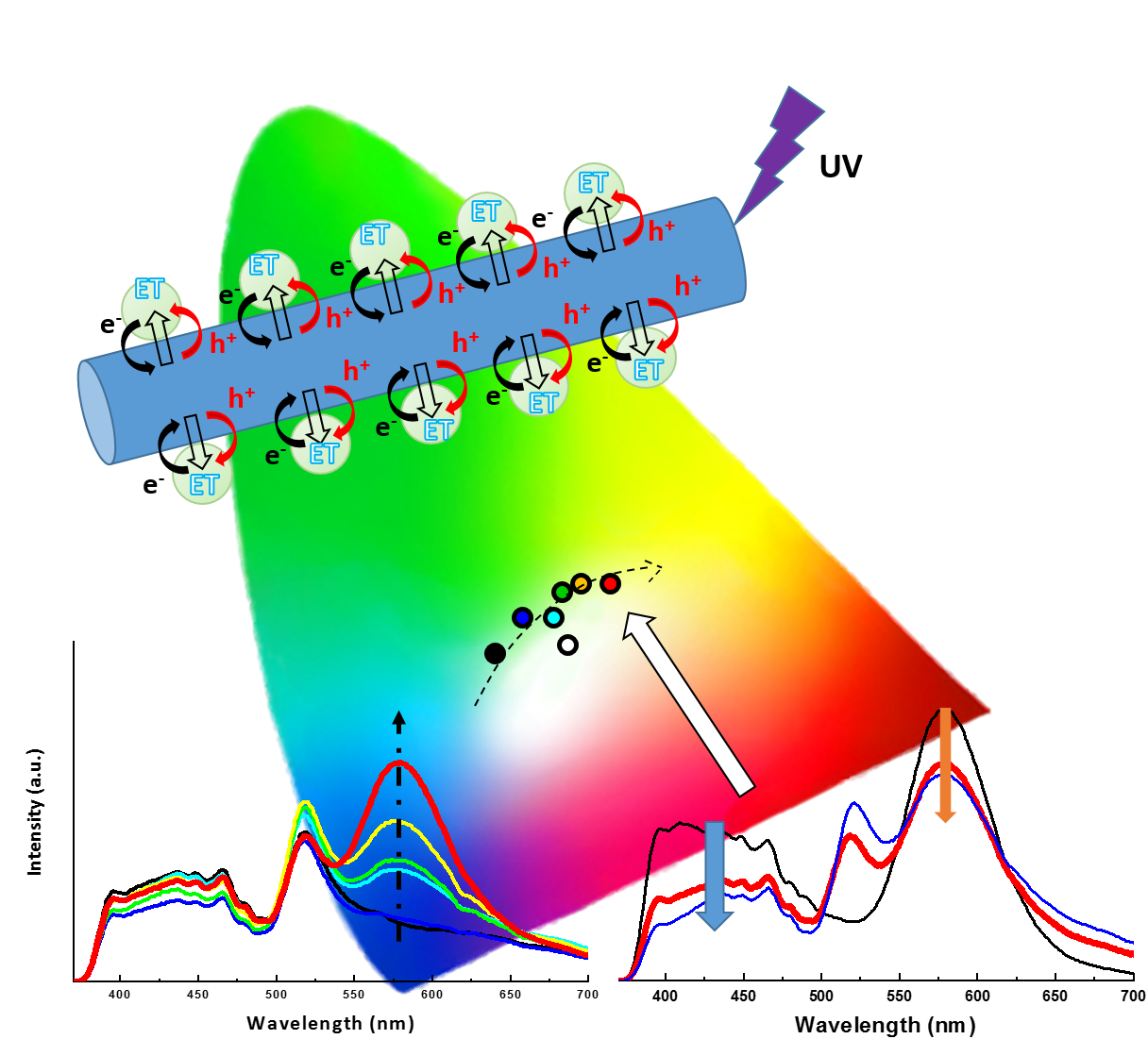
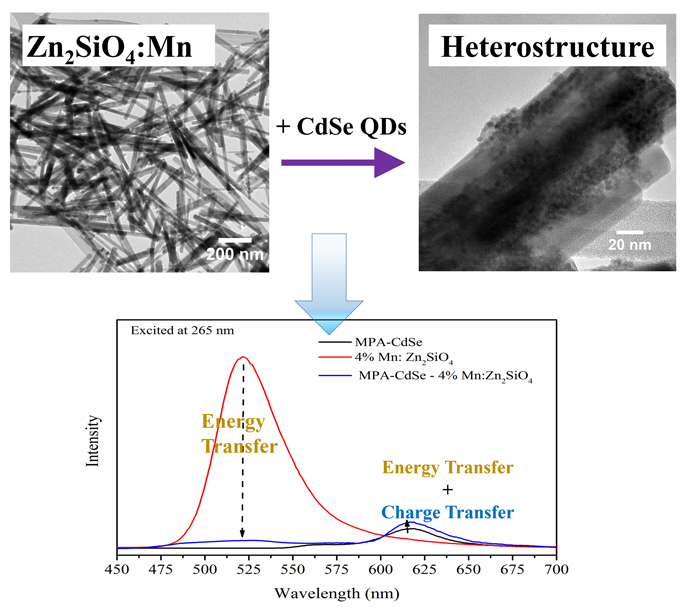
14. In this work, we have put forth a facile hydrothermal approach to synthesize an array of one-dimensional (1D) Mn-doped Zn2SiO4 nanostructures. Specifically, we have probed and correlated the effects of controllable reaction parameters such as the pH and Mn dopant concentrations with the resulting crystal structures and morphologies of the products obtained. Based upon our results, we find that careful tuning of the pH versus the Mn dopant level gives rise to opposite trends with respect to the overall size of the resulting one-dimensional nanostructures. Significantly, we have highlighted the role of the Mn dopant ion concentration as a potentially generalizable reaction parameter in solution-based synthesis for controlling morphology and hence, the observed optical behavior. Indeed, such a strategy can be potentially generalized to systems such as but not limited to Mn-doped ZnS, CdS, and CdSe quantum dots (QD), which, to the best of our knowledge, denote promising candidates for a variety of optoelectronic applications. Specifically, we have carefully optimized the synthesis conditions in order to generate a series of chemically well-defined Mn-doped Zn2SiO4 not only possessing Mn concentrations ranging from 3% to 8% but also characterized by highly crystalline, monodisperse wire-like motifs measuring ~30 nm in diameter and ~ 700 nm in length. Optically, the photoluminescence signals associated with the 1D series yielded a volcano-shaped relationship between PL intensities and the Mn dopant level.
In additional experiments, we have immobilized CdSe quantum dots (QDs) onto the external surfaces of our as-synthesized Mn-doped Zn2SiO4 nanowires, in order to form novel composite heterostructures. The optical properties of the CdSe QD - Mn: Zn2SiO4 heterostructures have been subsequently examined. Our results have demonstrated the likely co-existence of both energy transfer and charge transfer phenomena between the two constituent components of our as-prepared composites. Specifically, when both components are photoexcited, both energy transfer and charge transfer were found to plausibly occur, albeit in opposite directions. When the CdSe QDs are excited alone for example, charge transfer probably takes place from the CdSe QDs to the dopant Mn2+ ions. We believe that our as-processed heterostructures are therefore promising as a tunable light-harvesting motif. Essentially, these materials have broadened the effective light absorption range for optical ‘accessibility’, not only through their incorporation of dopant-tunable Zn2SiO4 possessing complementary absorption properties to those of the QDs but also through their integration of CdSe QDs with size-tailorable optical behavior.
Ref.:Phys. Chem. Chem. Phys., 20(15), 10086-10099 (2018).