
Christopher Johnson
Professor
B.S., Butler University, 2005
Ph.D., University of California, San Diego, 2011
NSF Postdoctoral Fellow, Yale University, 2011-2013
Postdoctoral Associate, Yale University, 2013-2014
521 Chemistry
Phone: (631) 632-7577
Email: chris.johnson@stonybrook.edu
- Research Description
MULTIPLEXING LASER SPECTROSCOPY AND MASS SPECTROMETRY TO ADDRESS CHALLENGES IN ENERGY AND THE ENVIRONMENT
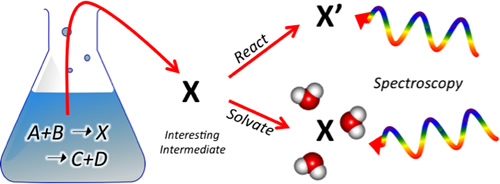
We are interested in resolving the molecular-level mechanisms underlying a broad range of chemical phenomena from new particle formation in the atmosphere to catalytic activity in solution and biofuel combustion, as well as understanding the active role that solvent (particularly water) plays in these reactions. Our contribution to these fields involves identifying and characterizing the reactive intermediates governing these chemical processes, which are often too short lived or in too low concentration to be observed using typical chemical analysis techniques. By harnessing and continuing to push forward new advances in mass spectrometry and ion trapping, we can create unique instruments that, in a very general way, isolate intermediates of interest directly from solution, chemically manipulate them through controlled gas-phase reactions, then interrogate them using a powerful and ever-expanding suite of spectroscopic (electronic and vibrational) and thermochemical techniques. Please visit the group site for more information on how these experiments are carried out.
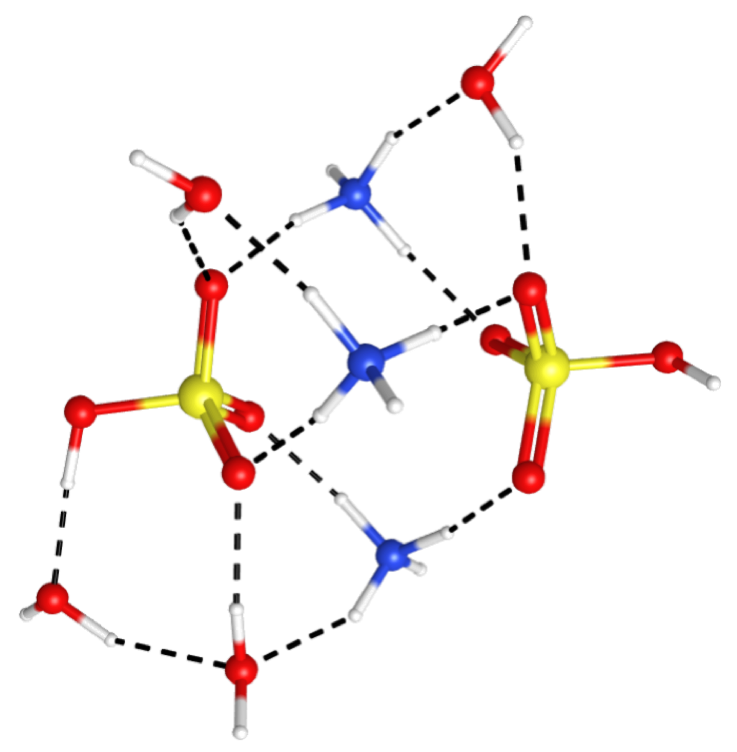
ATMOSPHERIC CHEMISTRY
New particle formation (the generation of particles directly from atmospheric vapors) is a nearly ubiquitous process in the ambient atmosphere, but it remains one of the most poorly understood. This is largely due to a lack of understanding of the critical chemical interactions taking place in these highly heterogeneous systems at the few-molecule to cluster level, or the sub-nanometer to nanometer range. We intend to fill this gap in understanding by generating seed clusters of 3-10 molecules, reacting them in a controlled way with vapors expected to play a role in particle growth, and using vibrational spectroscopy and thermochemistry to identify and characterize the critical intermolecular interactions responsible for controlling particle growth rates.
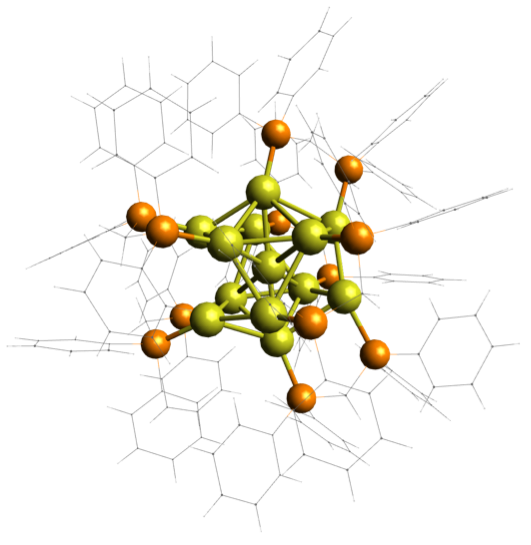
NANOSCIENCE
Metal nanoclusters are an interesting class of nanomaterials because they can, in some cases, be synthesized or purified with atomic precision, in that the exact number and types of atoms comprising them is exactly known. While their geometric structures can be determined using x-ray crystallography, their electronic structure, the shapes and energies of the molecular orbitals in which their electrons reside, is much harder to study. Furthermore, only certain compositions can be purified, limiting the diversity of their chemistry available for study. Our focus is to understand how the chemistry of these nanoclusters can be controlled and optimized to enhance their performance in a range of applications. We use mass spectrometry to purify impure samples, and record UV/vis absorption spectra of the now-pure sample inside the mass spec. This allows us to systematically vary their chemistry and reveal underlying rules governing their behavior.
CATALYSIS
We are interested in characterizing catalysts in the act of catalysis – specifically isolating the active species and trapping the active catalyst-reactant complex. This information can help to rigorously validate the proposed cycles for many catalysts, fine tuning our understanding of the overall mechanisms. We are also interested in what, if any, role individual solvent molecules may play in these processes, whether they be structural in nature or active participants in proton transfer, etc.
FUNDAMENTALS OF SPECTROSCOPY AND INTERMOLECULAR INTERACTIONS
Tying together all of our interests are open questions in physical chemistry regarding the non-trivial ways in which molecules interact, and the spectroscopic ramifications of these interactions. Ultimately, we find that in order to properly analyze the results in the projects above, we must first stop to understand some new phenomenon upon which we stumble. This interplay between fundamentals and applications always keeps this work exciting!
- Selected Publications
Kreinbihl, J. J., Frederiks, N. F., Waller, S. E., Yang, Y., and Johnson, C. J., "Establishing the structural motifs present in small ammonium and aminium bisulfate clusters of relevance to atmospheric new particle formation," J. Chem. Phys., 153, 034307 (2020). link highlight [Editor's Pick]
Cirri, A., Morales Hernandez, H., and Johnson, C. J., "High Precision Electronic Spectroscopy of Ligand-Protected Gold Nanoclusters: Effects of Composition, Environment, and Ligand Chemistry," J. Phys. Chem. A, 124, 1467-1479 (2020). link [Feature Article]
Cirri, A., Morales Hernandez, H., and Johnson, C. J., "Hydride, Chloride, and Bromide Show Similar Electronic Effects in the Au9(PPh3)83+ Nanocluster," Chem. Commun., 56, 1283-1285 (2020). link
Waller, S. E., Yang, Y., Castracane, E., Kreinbihl, J. J., Nickson, K. A., and Johnson, C. J., "Electrospray Ionization-Based Synthesis and Validation of Amine- Sulfuric Acid Clusters of Relevance to Atmospheric New Particle Formation," J. Am. Soc. Mass Spectrom., 30, 2267-2277 (2019). link
Racow, E. E.,* Kreinbihl, J. J.,* Cosby, A. G., Yang, Y., Pandey, A., Boros, E., and Johnson, C. J., "General Approach to Direct Measurement of the Hydration State of Coordination Complexes in the Gas Phase: Variable Temperature Mass Spectrometry," J. Am. Chem. Soc., 141, 14650-14660 (2019). link *Co-first authors
Cirri, A., Morales Hernandez, H., Kmiotek, C., and Johnson, C. J., "Systematically tuning the electronic structure of gold nanoclusters through ligand derivatization," Angew. Chem. Int. Ed., 58, 13828-13822 (2019). link Nat. Rev. Chem. highlight
Yang, Y. and Johnson, C. J., "Hydration Motifs of Ammonium Bisulfate Clusters of Relevance to Atmospheric New Particle Formation," Faraday Discuss., 217, 47-66, (2019). link
Yang, Y., Waller, S. E., Kreinbihl, J. J., and Johnson, C. J., "Direct Link between Structure and Hydration in Ammonium and Aminium Bisulfate Clusters Implicated in Atmospheric New Particle Formation," J. Phys. Chem. Lett., 9, 5647-5652 (2018). link
Waller, S. E., Yang, Y., Castracane, E., Racow, E. E., Kreinbihl, J. J., Nickson, K. A., and Johnson, C. J., "The Interplay between Hydrogen Bonding and Coulombic Forces in Determining the Structure of Sulfuric Acid-Amine Clusters," J. Phys. Chem. Lett., 9, 1216-1222 (2018). link
