
Robert B. (Barney) Grubbs
Professor
B.A. Pomona College, 1993
M.S. Cornell University (w/ J. Fréchet), 1995
Ph.D. Cornell University (w/ J. Fréchet), 1998
Postdoctoral research at the University of Minnesota (w/ F. Bates)
775 Chemistry
Phone: (631) 632-7911
Email: robert.grubbs@stonybrook.edu
- Research Description
My research group investigate a diversity of questions situated about the common ground shared by polymer, organic, and materials chemistry. With guidance from physics, theory, and engineering, we design, synthesize, and characterize organic materials with a focus on polymer-based materials and the designed inclusion of functional within these materials. Whenever possible, we try to work with systems capable of assembling into aggregates with a greater degree of structure. Such assemblies, many inspired by biological systems, can exhibit novel properties with applications in materials for medical and energy applications.
While there is rarely equity between different polymerization mechanisms in accessible structures, we aim to be inclusive toward all polymerization methods to achieve a diversity of polymer architectures. Controlled free radical, organometallic, organocatalytic, anionic, and cationic polymerization methods, either alone or in combination with one another, can provide access to many possible polymeric structures, and many techniques of organic chemistry are applicable to the modification of these polymers for the preparation of an even larger variety of materials. We use these techniques to realize specifically designed polymeric architectures. Synthesis of these materials is the major focus of the research program, and students will gain experience in many synthetic techniques. A number of techniques for characterization of new materials by established methods (i.e., small-angle scattering, electron microscopy, etc.) at both the molecular level and at longer length scales are also used.
Representative research projects being undertaken are outlined below. We typically use living or controlled polymerization techniques in combination with initiator and/or monomer synthesis for the creation of novel polymers that should exhibit interesting self-assembly behavior in solution and in bulk.
- Controlling Aldehyde Polymerization
- Stimulus-Induced Morphological Transformation of Ternary Copolymer Micelles
- Incorporation of Metallic Nanoparticles into Polymeric Matrices
- Conjugated Molecules and Polymers for Electronic Applications
Controlling aldehyde polymerization
Chain polymerization of carbonyl compounds results in polymers with acetal linkages in the backbone that have potential as degradable or recyclable materials. The wide variety of aldehydes and ketones that occur naturally or through sustainable transformations of naturally occurring compounds suggests that they also have promise as sustainable materials. There are many challenges associated with purifying aldehyde and ketone monomers and with controlling their polymerization. Glyoxylate esters are one such class of monomers that can be polymerized by treatment with bases, but monomer purification is a critical issue and the polymerization mechanism has not been studied in detail. We have demonstrated the controlled polymerization of glyoxylate esters from alcohols, thiols, and hydroxy-terminated macroinitiators (Figure 1, Figure 2) (Hewitt & Grubbs 2021). This has enabled the preparation of a range of block copolymers with degradable polyglyoxylate blocks, including polymers that form hydrogels in collaboration with Prof. S. Bhatia (Yin et al. 2022). On-going efforts are being directed toward expanding these polymerization methods to include other monomers and investigating the sustainability of these methods.
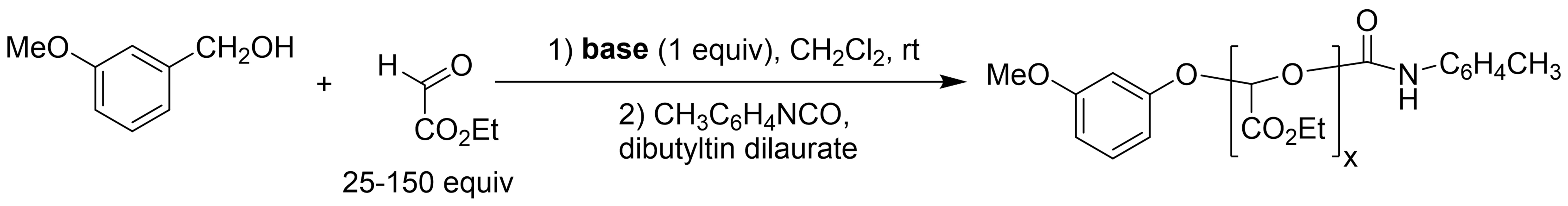
Figure 1.Polymerization of ethyl glyoxylate from alcohol initiators with organic bases (e.g., triethylamine). (Hewitt & Grubbs 2021)
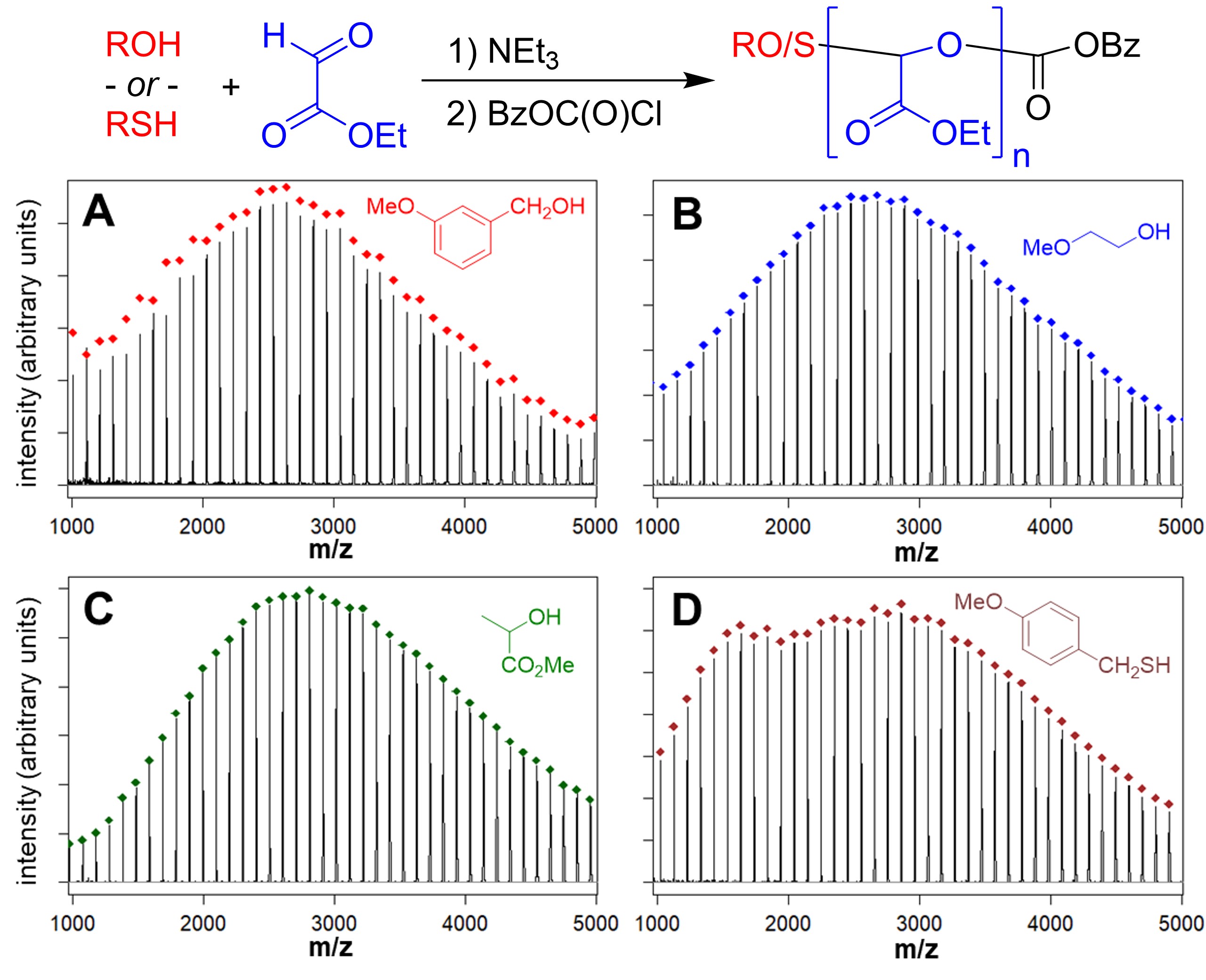
Figure 2.MALDI-TOF MS data for poly(ethyl glyoxylate) prepared with different alcohol and thiol initiators. (Hewitt & Grubbs 2021)
STIMULUS-INDUCED MORPHOLOGICAL TRANSFORMATIONS OF TERNARY COPOLYMER MICELLES
The micellar form which amphiphilic block copolymers adopt in water is strongly governed by the relative volumes of the hydrophobic and hydrophilic blocks. We have demonstrated that ABC triblock copolymers comprising one hydrophobic block, one stimulus-responsive block, and one hydrophilic block can assemble into different structures depending upon the presence of specific stimuli (Figure 3).(Grubbs & Sun 2013; Cai et al. 2011; Sundararaman et al. 2008; Wegrzyn et al. 2005; Aubrecht & Grubbs 2005) Poly(ethylene oxide)-block-poly(N-isopropylacrylamide)-block-poly(isoprene) (PEO-PNIPA-PI) copolymers that undergo a thermally induced reorganization in water from small spherical micelles (~10-20 nm diameter) to much larger spherical vesicles (~200 nm in diameter) have been synthesized, though the transformation from micelles to vesicles takes approximately four weeks (Figure 4).(Sundararaman et al. 2008)
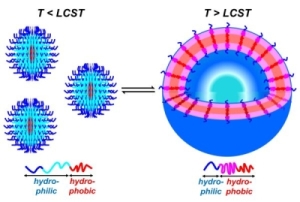
Figure 3. Representation of small polymer assemblies formed at low temperatures (T < LCST) and larger hollow assemblies formed at higher temperatures (T > LCST). (Cai et al. 2011)
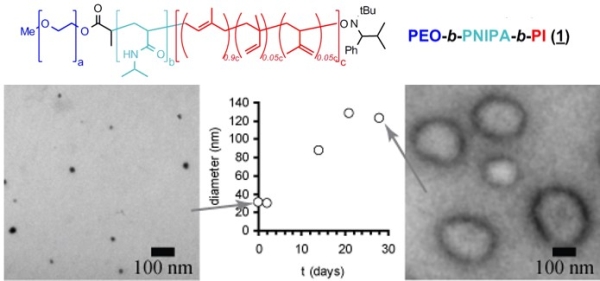
Figure 4. Poly(ethylene oxide)-block-poly(N-isopropylacrylamide)- block-poly(isoprene) structure (above); transmission electron micrographs of polymer assemblies in water at low (a) and high (b) temperatures; and evolution of assembly diameter over time by dynamic light scattering. (Sundararaman et al. 2008)
Much more rapid structural transformations were demonstrated by a second class of poly(ethylene oxide)-block-poly(butylene oxide-stat-ethylene oxide)-block-poly(isoprene) (PEO-PBOEO-PI) copolymers. Our working hypothesis is that the absence of strong interchain interactions in the responsive block in these systems facilitates a more rapid transformation than that observed for the acrylamide-based PEO-PNIPA-PI system (Figure 5). (Cai et al. 2011)
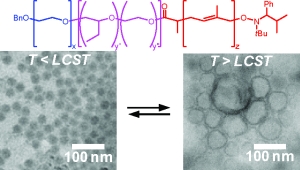
Figure 5. Poly(ethylene oxide)-block-poly(butylene oxide-stat-ethylene oxide)-block-poly(isoprene) (PEO-PBOEO-PI) structure (top) and transmission electron micrographs of polymer assemblies in water at room temperature (bottom left) and after 48 h at 70 °C (bottom right). (Cai et al. 2011)
We are currently investigating two new related classes of these responsive polymers. Poly(ethylene oxide)-block-poly(N,N-diethylacrylamide)-block-poly(N,N-dibutylacrylamide) (PEO-b-PDEAm-b-PDBAm) have been designed as an example of an acrylamide-based system (like PNIPA) that cannot form hydrogen bonds with other PDEA chains (like PBOEO) (Figure 6). Assemblies of these polymer do appear to undergo rapid transformations upon heating. Analogous polymers with pendent photo-cross-linkable azide functional groups (PEO-b-PDEAm-b-PDBAm*) have also been prepared to facilitate the trapping of polymer assemblies at different temperatures during the transformation process. At slightly higher concentrations (5-10 wt%), PEO-b-PDEAm-b-PDBAm copolymers that undergo a spherical-to-cylindrical micelle transition reversibly form hydrogels. (Sun et al. 2017)
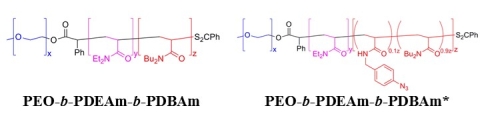
Figure 6. Structures of polyacrylamide-based responsive triblock copolymers.
INCORPORATION OF METALLIC NANOPARTICLES INTO POLYMERIC MATRICES
We have investigated multiblock copolymers wherein different blocks are used to specifically stabilize the formation of ordered arrays of nanoparticles composed of different metals.
We have shown that alkyne functional block copolymers can be prepared by direct controlled radical polymerization of alkyne-functional monomers or by the coupling of terminal alkynes to polymers containing 4-bromostyrene repeating units.(Sessions et al., 2007) Cobalt carbonyl (and potentially other inorganic or organometallic species) can be selectively reacted with the alkyne-functional block to give hybrid composite materials with cobalt units spatially patterned on the nanometer scale (Figure 7).(Mîinea et al., 2004; Sessions et al., 2005; Grubbs 2005) The heating of these materials leads to the formation of cobalt metal within the patterned domains.
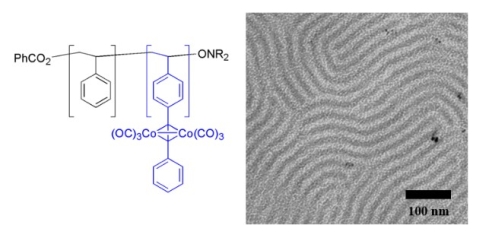
Figure 7. Polystyrene-block-poly(4-phenylethynylstyrene) structure (left) and typical micrograph of bulk diblock copolymer structure after treatment with cobalt carbonyl. (Mîinea et al., 2004)
Later work was directed at mapping out the phase behavior of cobalt carbonyl adducts of polystyrene-block-poly(4-phenylethynylstyrene) (PS-PPES) (Jiang et al. Macromolecules 2016) and poly(ethylene oxide)-block-PPES and providing a better understanding of the thermolysis process that affords cobalt metal and the selectivity of inclusion of metal particles within specific polymer domains. We have also investigated the formation of hydrogels from cobalt carbonyl adducts of PPES-block-poly(ethylene oxide)- block-PPES triblock copolymers (Jiang et al. J. Am. Chem. Soc. 2016, videos).
CONJUGATED MOLECULES AND POLYMERS FOR ELECTRONIC APPLICATIONS
We have investigated the preparation of a diverse range of new conjugated molecules and macromolecules for electronic applications, especially in organic solar cells in collaboration with the Electronic Materials Group at the Center for Functional Nanomaterials at Brookhaven National Laboratory.
As part of a project designed to probe the potential utility of replacing sulfur with a more inclusive set of higher molecular weight calchogens (selenium, tellurium), we developed methods for using ipso-arylative coupling chemistry for the synthesis of π-conjugated molecules and macromolecules. These methods allow the use of organic monomers and offer advantages in terms of monomer stability (as compared to the boronic acid derivatives used in the Suzuki-Miyaura coupling) and toxicity (when compared with the alkyltin derivatives used in the Stille coupling). Photovoltaic devices based on small molecule absorbers with a benzochalcogenophene-diketopyrrolopyrrole-benzochalcogenophene structure in the benzothiophene, benzoselenophene, benzotellurophene series showed a maximum power conversion efficiency of 5.8% for the benzoselenophene derivative (Figure 8).(Park et al., Chem Commun 2014) While the benzotellurophene derivative showed a lower bandgap, we suspect side reactions resulting from instability of the benzotellurophene moiety reduced the overall efficiency.
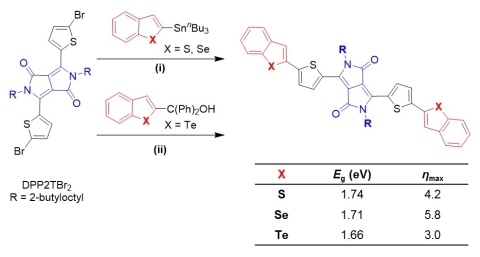
Figure 8. Synthesis of benzochalcogenophene/diketopyrrolopyrrole donor-acceptor-donor molecules and measured bandgaps and maximum power conversion efficiencies (ηmax) in bulk heterojunction solar cells with PCBM. (Park et al., Chem Commun 2014)
Tellurophene-containing analogues of the conjugated polymer PDPP3T were prepared by a similar ipso-arylative coupling method (Figure 9) (Park et al., Ang Chem Int Ed 2014). Current generation at wavelengths as high as 1000 nm was observed from bulk heterojunction photovoltaic devices prepared from these polymers and PC71BM, with a maximum power conversion efficiency of 4.4%.
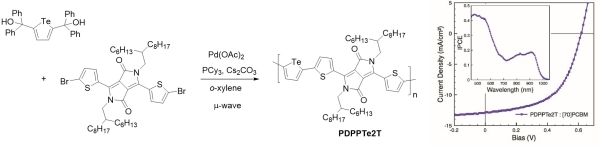
Figure 9. Synthesis of PDPPTe2T by ipso-arylative coupling (left). Typical I-V curve and IPCE spectrum (inset) for a bulk heterojunction solar cell prepared from PDPPTe2T and PC71BM (right). (Park et al., Ang Chem Int Ed 2014)
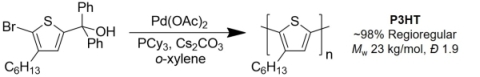
While the stability of tellurophene-based molecules and macromolecules appears to limit their efficiency in photovoltaic devices, we plan to continue to explore the use of ipso-arylative coupling methods for the preparation of a range of π-conjugated motifs for optoelectronic applications. Preliminary efforts have shown that aromatic monomers bearing both diarylcarbinol and bromine groups, such as 2-bromo-3-hexyl-5-(hydroxydiphenylmethyl)thiophene can undergo ipso-arylative polymerization to afford P3HT with high regioregularity (Scheme 1) (Shih et al., Polym Chem 2018).
Scheme 1.
This material is based upon work supported by the National Science Foundation under Grants CHE-2411615, CHE-1904932, DMR-1905547, CHE-1506822, DMR-0239697, and DMR-0804792. Any opinions, findings and conclusions or recomendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation (NSF).
- Selected Publications
Yin, X.; Hewitt, D. R. O.; Zheng, B.; Yu, X.; Carr, A.; Grubbs, R. B.; Bhatia, S. R. "Aqueous Assembly and Hydrogel Rheology of Sustainable Glyoxylate-based Copolymers." ACS Applied Polymer Materials2022, 4, 5493-5500. (doi: 10.1021/acsapm.2c00542)
Yin, X.; Hewitt, D. R. O.; Zheng, B.; Quah, S. P.; Stanley, C. B.; Grubbs, R. B.; Bhatia, S. R. “Effect of stereochemistry on nanoscale assembly of ABA triblock copolymers with crystallizable blocks.” Polymer2021, 223, 123683. (doi: 10.1016/j.polymer.2021.123683)
Hewitt, D. R. O.; Grubbs, R. B. “Amine-catalyzed chain polymerization of ethyl glyoxylate from alcohol and thiol initiators.” ACS Macro Letters 2021, 10, 370-374. (doi: 10.1021/acsmacrolett.0c00865)
Subramanian, A.; Doerk, G.; Kisslinger, K.; Yi, D. H.; Grubbs, R. B.; Nam, C.-Y. “Priming-Free, Direct Infiltration Synthesis of Electroactive ZnO Nanomesh Using Pyridine-Based Self-Assembled Block Copolymer Thin Films.” Nanoscale2019, 11, 9533-9546. (doi: 10.1039/C9NR00206E)
Yin, X.; Hewitt, D. R. O.; Preston, A. N.; Heroux, L. A.; Agamalian, M. M.; Quah, S. P.; Zheng, B.; Smith, A. J.; Laughlin, S. T.; Grubbs, R. B.; Bhatia, S. R. “Hierarchical assembly in triblock gels with crystalline domains: Evidence from ultra-small-angle neutron scattering and confocal microscopy.” Colloids Surf. B2019, 180, 102-109. (doi: 10.1016/j.colsurfb.2019.04.031)
Yi, D. H.; Nam, C.-Y.; Doerk, G.; Black, C. T.; Grubbs, R. B. “Infiltration synthesis of diverse metal oxide nanostructures from epoxidized diene–styrene block copolymer templates.” ACS Appl. Polym. Mater. 2019, 1, 672-683. (doi: 10.1021/acsapm.8b00138)
Shih, F.-Y.; Choi, D.; Wu, Q.; Nam, C.-Y.; Grubbs, R. B. “ipso-Arylative Ring-opening Polymerization as a Route to Electron-deficient Conjugated Polymers.” Angew. Chem. Int. Ed.2019, 58, 288-291. (doi: 10.1002/anie.201809610)
Yin, X.; Hewitt, D. R. O.; Quah, S. P.; Zheng, B.; Grubbs, R. B.; Bhatia, S. R. “Impact of Stereochemistry on Rheology and Nanostructure of PLA-PEO-PLA Triblocks: Stiff Gels at Intermediate L/D-lactide Ratios.” Soft Matter2018, 14, 7255-7263. (doi: 10.1039/C8SM01559G)
Shih, F.-Y.; Tian, S.; Gallagher, N.; Park, Y. S.; Grubbs, R. B. “ipso-Arylative polymerization as a route to π-conjugated polymers: Synthesis of poly(3-hexylthiophene).” Polym. Chem. 2018, 9, 3223-3231. (doi: 10.1039/C8PY00605A)
Wu, T.; Cai, Y.; Zhao, X.; Ngai, C. K.; Chu, B.; Hsiao, B.; Hadjiargyrou, M.; Grubbs, R. B. “Synthesis and Characterization of Poly(ethylene oxide)/polylactide/polylysine Tri-arm Star Copolymers for Gene Delivery.” J. Polym. Sci., Part A: Polym. Chem.2018, 56, 635-644. (doi: 10.1002/pola.28938)
Grubbs, R. B.; Grubbs, R. H. “50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules.” Macromolecules2017, 50, 6979-6997. (doi: 10.1021/acs.macromol.7b01440)
Ye, X.; Kestell, J.; Kisslinger, K.; Liu, M.; Grubbs, R. B.; Boscoboinik, J. A.; Nam, C.-Y. “Effects of residual solvent molecules facilitating the infiltration synthesis of ZnO in a non-reactive polymer.” Chem. Mater.2017, 29, 4535-4545. (doi: 10.1021/acs.chemmater.7b01222)
Sun, Z.; Tian, Y.; Hom, W. L.; Gang, O.; Bhatia, S. R.; Grubbs, R. B. “Translating thermal response of triblock copolymer assemblies in dilute solution to macroscopic gelation and phase separation.” Angew. Chem.2017, 56, 1491-1494. (link)
Jiang, B.; Hom, W.; Chen, X.; Yu, P.; Pavelka, L.; Kisslinger, K.; Parise, J. B.; Bhatia, S.; Grubbs, R. B. “Magnetic Hydrogels from Alkyne/Cobalt Carbonyl-functionalized ABA Triblock Copolymers.” J. Am. Chem. Soc.2016, 138, 4616-4625. (link)
Jiang, B.; Nykypanchuk, D.; Endoh, M. K.; Chen, X.; Qian, B.; Kisslinger, K.; Koga, T.; Parise, J. B.; Grubbs, R. B. “Phase behavior of alkyne-functionalized styrenic block copolymer/cobalt carbonyl adducts and in situ formation of magnetic nanoparticles by thermolysis.” Macromolecules2016, 49, 853-865. (link)
Rowan, S.; Barner-Kowollik, C.; Klumperman, B.; Gaspard, P.; Grubbs, R. B.; Hillmyer, M.; Hutchings, L.; Mahanthappa, M.; O’Reilly, R.; Ouchi, M.; Sawamoto, M.; Lodge, T. “Discussion on ‘Aperiodic Polymers’.” ACS Macro Letters2016, 5, 1-3. (link)
Song, X.; Ma, Q.; Cai, Z.; Tanaka, R.; Shiono, T.; Grubbs, R. B. “Facile synthesis of novel polyethylene-based A-B-C block copolymers containing poly(methyl methacrylate) using a living polymerization system.” Macromol. Rapid Commun.2016, 37, 227-231. (link)
Yang, R.; Su, Y.; Aubrecht, K. B.; Wang, X.; Ma, H.; Wang, R.; Grubbs, R. B.; Hsiao, B. S.; Chu, B. “Thiol-functionalized Chitin Nanofibers for As(III) Adsorption.” Polymer2015, 60, 9-17. (link)
Park, Y.S.; Wu, Q.; Nam, C.-Y.; Grubbs, R.B. “Polymerization of tellurophene derivatives via microwave-assisted palladium-catalyzed ipso-arylative polymerization.” Angew. Chem. Int. Ed.2014, 53, 10691-10695. (link)
Park, Y.S.; Kale, T.S.; Nam, C.-Y.; Choi, D.; Grubbs, R. B. “Effects of heteroatom substitution in conjugated heterocyclic compounds on photovoltaic performance: from sulfur to tellurium.” Chem. Commun.2014, 50, 7964-7967. (link)
Yang, R.; Aubrecht, K. B.; Ma, H.; Wang, R.; Grubbs, R. B.; Hsiao, B. S.; Chu, B. “Thiol-modified Cellulose Nanofibrous Composite Membranes for Chromium (VI) and Lead (II) Adsorption.” Polymer2014, 55, 1167-1176. (link)
Grubbs, R. B.; Sun, Z. “Shape-changing polymer assemblies.” Chem. Soc. Rev.2013, 42, 7436-7445. (link)
Kamcev, J.; Germack, D. S.; Nykypanchuk, D.; Grubbs, R. B.; Nam, C.-Y.; Black, C. T. “Chemically Enhancing Block Copolymers for Block-Selective Synthesis of Self-Assembled Metal Oxide Nanostructures.” ACS Nano2013, 7, 339-346. (link)
Nam, C.-Y.; Qin, Y.; Park, Y. S.; Hliang, H.; Lu, X.; Ocko, B. M.; Black, C. T. Grubbs, R. B. “Photocrosslinkable azide-functionalized polythiophene for thermally stable bulk heterojunction solar cells.” Macromolecules2012, 45, 2338-2347. (link)
Grubbs, R. B. “Nitroxide-Mediated Radical Polymerization: Limitations and Versatility.” Polym. Rev.2011, 51, 104-137. (link)
Cai, Y.; Aubrecht, K. B.; Grubbs, R. B. “Thermally-induced Changes in Amphiphilicity Drive Reversible Restructuring of Assemblies of ABC Triblock Copolymers with Statistical Polyether Blocks.” J. Am. Chem. Soc.2011, 133, 1058-1065. (link)
Greene, A.; Grubbs, R. B. “Synthesis and Evaluation of an Ester-Functional Alkoxyamine and Corresponding Nitroxide-Fidelity Studies.” J. Polym. Sci., Part A: Polym. Chem2009, 47, 6342-6352. (link)
Greene, A.; Grubbs, R. B. “Synthesis and Evaluation of N-Phenylalkoxyamines for Nitroxide-Mediated Polymerization.” Macromolecules2009, 42, 4388-4390. (link)
Greene, A.; Grubbs, R. B. “Current Methods for N-Alkoxyamine Synthesis.” ACS Symposia2009, 1024 (Controlled/Living Radical Polymerization: Progress in RAFT, DT, NMP & OMRP), 81-93. (link)
Sundararaman, A.; Stephan, T.; Grubbs, R. B. “Reversible Restructuring of Aqueous Block Copolymer Assemblies through Stimulus-Induced Changes in Amphiphilicity.” J. Am. Chem. Soc.2008, 130, 12264-12265. (link)
Grubbs, R. B. “Roles of Polymer Ligands in Nanoparticle Stabilization.” Polym. Rev.2007, 47, 197-215. (link)
Sessions, L. B.; Cohen, B. R.; Grubbs, R. B. “Alkyne-Functional Polymers through Sonogashira Coupling to Poly(4-Bromostyrene).” Macromolecules2007, 40, 1926-1933. (link)
Xia, Q.; Grubbs, R. B. “In Situ Generation of Nitroxide from Alkoxyamines for Controlled Acrylate Polymerization.” J. Polym. Sci., Part A: Polym. Chem.2006, 44, 5128-5136. (link)
Aubrecht, K. B.; Grubbs, R. B. "Synthesis and Characterization of Thermoresponsive Amphiphilic Block Copolymers Incorporating a Poly(Ethylene oxide-stat-Propylene oxide) Block." J. Polym. Sci., Part A: Polym. Chem.2005, 43, 5156-5167. (link)
Grubbs, R. B. "Hybrid Metal-Polymer Composites from Functional Block Copolymers." J. Polym. Sci., Part A: Polym. Chem.2005, 43, 4323-4336. (link)
Wegrzyn, J. K.; Stephan, T.; Lau, R. N.; Grubbs, R. B. "Preparation of poly(ethylene oxide)-block-polyisoprene by nitroxide-mediated free radical polymerization from PEO macroinitiators." J. Polym. Sci., Part A: Polym. Chem.2005, 43, 2977-2984.(link)
Sessions, L. B.; Mîinea, L. A.; Ericson, K. D.; Glueck, D. S.; Grubbs, R. B. "Alkyne-functional Homopolymers and Block Copolymers through Nitroxide-Mediated Free Radical Polymerization of 4-(Phenylethynyl)styrene." Macromolecules2005, 38, 2116-2121. (link)
Grubbs, R. B.; Wegrzyn, J. K.; Xia, Q. "One-step synthesis of alkoxyamines for nitroxide-mediated radical polymerization." Chem. Comm.2005, 80-82. (link)
Mîinea, L. A.; Sessions, L. B.; Ericson, K. D.; Glueck, D. S.; Grubbs, R. B. "Phenylethynylstyrene-Cobalt Carbonyl Block Copolymer Composites." Macromolecules2004, 37, 8967-8972. (link)
