Tadashi Honda
Research Professor
B.S., 1974, M.S., 1976, Ph.D., 1979, The University of Tokyo.
Suntory Institute for Biomedical Research 1979–1991.
Chief Senior Researcher, 1991–1995, Central Pharmaceutical Research Institute at Japan
Tobacco Inc.
Research Faculty [Research Associate Professor (2005)], 1995–2010, Dartmouth College.
Research Professor, 2010–present, Stony Brook University
743 Chemistry
Phone: (631) 632-7162
Email: tadashi.honda@stonybrook.edu
- Research Description
Drug discovery of new anti-inflammatory and cytoprotective agents as well as the new chemistry that is derived from their syntheses and modifications has been the primary objectives of Dr. Honda's research program.
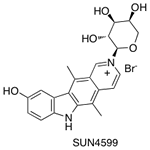
Dr. Honda was involved in the discovery of anti-cancer drugs based on natural product models over the past two decades in Japan. Amongst several anti-cancer drug candidates that he invented during his pharmaceutical career, 2α-L-arabinopyranosyl-9-hydroxyellipticinium bromide (SUN4599) was evaluated in phase II clinical trials for the treatment of solid tumors, but failed due to hepatoxicity.
Dr. Honda has been engaged in the development of new anti-inflammatory and cytoprotective agents by modifications of naturally occurring pentacyclic triterpenoids at Dartmouth College over the past 15 years. Amongst these new semisynthetic triterpenoids, bardoxolone methyl (BARD), which potently activates the cytoprotective Keap1/Nrf2/ antioxidant response element (ARE) pathway, was expected to be the first in class drug for the treatment of diabetic nephropathy. In a multi–center, double–blind, placebo–controlled phase 2b clinical trial, patients treated with BARD experienced a significant increase in estimated glomerular filtration rate (eGFR), compared with no change in the placebo group. Currently, BARD is being evaluated in phase 2 clinical trials for the treatment of pulmonary arterial hypertension (PAH) in the United States and diabetic nephropathy in Japan.
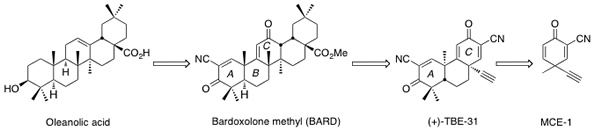
Mechanism studies suggest that BARD regulates various proteins regarding inflammation and carcinogenesis, including Keap1, IKKb, and JAK1, to name a few, by reversible Michael addition between the nonenolizable cyanoenone in ring A of BARD and the SH groups of cysteine moieties on these proteins. On the basis of the information that essential pharmacophores are nonenolizable cyanoenones, Dr. Honda designed tricyclic compounds containing nonenolizable cyanoenones which give reversible Michael adduct with a SH group. It has been shown that they are also potent activators of the Keap1/Nrf2/ARE pathway. Among such synthetic tricycles, TBE-31 with an ethynyl group shows remarkable features in various in vitro and in vivo bioassays related to inflammation and carcinogenesis, which BARD does not. Thus, TBE-31 is expected to be a second generation of therapeutic for the treatment of diabetic nephropathy as a back-up compound of BARD. His research group has already established an improved synthesis of TBE-31, which is suitable for a large scale production at low cost. The toxicological evaluation of TBE-31 has been started in collaboration with Dr. Dinkova-Kostova (University of Dundee) in the United Kingdom and Dr. Anna-Liisa Levonen (University of Eastern Finland) in Finland.
Novel monocyclic cyanoenones (MCEs), which are derived from rings A and C of TBE-31, display unique and interesting features with respect to reactivity (as Michael acceptors) and biological potency. In a preliminary set of MCEs, one of the most reactive Michael acceptor is MCE-1 but the addition is reversible. For induction of the phase 2 cytoprotective enzyme NQO1 in Hepa 1c1c murine hepatoma cells, MCE-1 demonstrates the highest potency (CD value: 22 nM). This high potency of MCE-1 is really striking because sulforaphane, a Keap1/Nrf2/ARE activator, which is widely used by many research groups worldwide and in many disease models related to inflammation and cancer, has a CD of 200 nM. Currently, Dr. Honda’s group is exploring entirely new anti-inflammatory and cytoprotective agents containing MCE-1 as a warhead in collaboration with Dr. Dale Mierke (Dartmouth College).
- Selected Publications
- Knatko, E. V.; Ibbotson, S. H.; Zhang, Y.; Higgins, M.; Fahey,J. W.; Talalay, P.; Dawe, R. S.; Ferguson,J.; Huang,J. T.-J.; Clarke, R.; Zheng, S.; Saito, A.; Kalra, S.; Benedict, A. L.; Honda, T.; Proby,C. M.; Dinkova-Kostova, A. T. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev. Res. 2015, 8, 475–486.
- Li, W.; Zheng, S.; Higgins, M.; Zoete, V.; Morra, R. P. Jr.; Mendis, A.; Chien, C.- W.; Ojima, I.; Mierke,⊥ New monocyclic, bicyclic, and tricyclic ethynylcyanodienones as activators of the Keap1/Nrf2/antioxidant response element pathway. J. Med. Chem. 2015, 58, 4738–4748.
- Abeti, R.; Uzun, E.; Renganathan, I.; Honda, T.; Mark A. Pook, M. A.; Giunti, P. Targeting lipid peroxidation and mitochondrial imbalance in Friedreich’s ataxia. Pharmcol. Res. 2015, 99, 344–350.
- Kostov, R. V.; Knatko, E. V.; McLaughlin, L. A.; Henderson, C. J.; Huang, J. T.- J.; Zheng S.; Honda, T.; Dinkova-Kostova, A. T. Pharmacokinetics and pharmacodynamics of orally administered acetylenic tricyclic bis(cyanoenone), a highly potent Nrf2 activator with a reversible covalent mode of action. Biochem. Biophys. Res. Commun. 2015, 465, 402–407.
- Chan, E.; Saito, A.; Honda, T.; Di Guglielmo, G. M. The acetylenic tricyclic bis(cyanoenone), TBE-31 inhibits non–Small cell lung cancer cell migration through direct binding with actin. Cancer Prev. Res. 2014, 7, 727–737. This article was featured on the cover of the issue.
- Copple, I. M.; Shelton, L. M.; Walsh, J.; Kratschmar, D. V.; Lister, A.; Odermatt, A.; Goldring, C. E.; Dinkova-Kostova, A. T.; Honda, T.; Park, B. K. Chemical tuning enhances both potency toward Nrf2 and in vitro therapeutic index of triterpenoids. Tox. Sci. 2014, 140, 462–469.
- Zheng, S.; Huang, J. T.-J.; Knatko, E. V.; Sharp, S.; Higgins, M.; Ojima, I.; Dinkova-Kostova, A. T.; Honda, T. Synthesis of 13C2 cytoprotective tricyclic bis(cyanoenone) ([13C2 quantification by stable isotope dilution LC-MS method. J. Label. Compd. Radiopharm. 2014, 57, 606–610.
- Saito, A.; Higgins, M.; Zheng, S.; Li, W.; Ojima, I.; Dinkova-Kostova, A. T.; Honda, T. Synthesis and biological evaluation of biotin conjugates of (±)-(4bS,8aR,10aS)-10a-ethynyl-4b,8,8-trimethyl-3,7-dioxo-3,4b,7,8,8a,9,10,10a-octahydro-phenanthrene-2,6-dicarbonitrile, an activator of the Keap1/Nrf2/ARE pathway, for the isolation of its protein targets. Bioorg. Med. Chem. Lett. 2013, 23, 5540–5543.
- Saito, A.; Zheng, S.; Takahashi, M.; Li, W.; Ojima, I.; Honda, T. An improved synthesis of a hydroxylmethyl tricyclic ketone from cyclohexanone, the key processes for the synthesis of a highly potent anti-inflammatory and cytoprotective agent. Synthesis 2013, 45, 3255–3258.
- Zheng, S.; Chowdhury, A.; Ojima, I.; Honda, T. Microwave-assisted Diels-Alder reactions between Danishefsky's diene and derivatives of ethyl α-(hydroxymethyl)acrylate. Synthetic approach towards a biotinylated anti-inflammatory monocyclic cyanoenone. Tetrahedron 2013, 69, 2052–2055.
- Kalra, S.; Knatko, E. V.; Zhang, Y.; Honda, T.; Yamamoto, Y.; Dinkova-Kostova A. T. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): implications for D. F.; Bensasson, R. V.;Dinkova-Kostova A. T.; Honda T. protection against UV radiation during thiopurine therapy. Cancer Prev. Res. 2012, 5, 973–981.
- Zheng, S; Laxmi, Y. R. S.; David, E; Dinkova-Kostova, A. T.; Katherine H. Shiavoni, K. H.; Ren, Y.; Zheng, Y.; Trevino, I.; Bumeister, R.; Ojima, I.; Wigley, W. C.; James J. B.; Mierke, D. F.; Honda, T. Synthesis, chemical reactivity as Michael acceptors, and biological potency of monocyclic cyanoenones, novel and highly potent anti-inflammatory and cytoprotective agents. J. Med. Chem. 2012, 55, 4837–4846.
- Honda, T.; Yoshizawa, H.; Sundararajan, C.; David, E.; Lajoie, M. j.; Favaloro, F. G. Jr.; Janosik, T.; Su, X.; Honda, Y.; Roebuck, B. D.; Gribble, G. W. Tricyclic Compounds Containing Non-enolizable Cyano Enones. A Novel Class of Highly Potent Anti-inflammatory and Cytoprotective Agents. J. Med. Chem. 2011, 21, 2188–2191.
- Dinkova-Kostova, A. T.; Talalay, P.; Sharkey, J.; Zhang, Y.; Holtzclaw, W. D.; Xiu Jun Wang, X. J.; David, E.; Schiavoni, K. H.; Finlayson, S.; Dale F. Mierke, D. F.; Honda, T. An Exceptionally Potent Inducer of Cytoprotective Enzymes: Elucidation of the Structural Features that Determine Inducer Potency and Reactivity with Keap1. J. Biol. Chem. 2010, 285, 33747–33755. This article was highlighted in a Spotlight section of Chemical Research in Toxicology.
- Liby, K.; Yore, M. M.; Roebuck B. D.; Baumgartner, K. J.; Honda, T.; Sundararajan, C.; Yoshizawa, H.; Gribble, G. W.; Williams, C. R.; Risingsong, R.; Royce, D. B.; Dinkova-Kostova, A. T.; Stephenson, K. K.; Egner, P. A.; Yates, M. S.; Groopman, J. D.; Kensler, T. W.; Sporn, M. B. A Novel Acetylenic Tricyclic Bis-(cyano enone) Potently Induces Phase 2 Cytoprotective Pathways and Blocks Liver Carcinogenesis Induced by Aflatoxin. Cancer Res. 2008, 68, 6727–6732.
- Honda, T.; Sundararajan, C.; Yoshizawa, H.; Su, X.; Honda, Y.; Liby, K. T.; Sporn, M. B.; Gribble, G. W. Novel Tricyclic Compounds Having Acetylene Groups at C-8a and Cyano Enones in Rings A and C: Highly Potent Anti-inflammatory and Cytoprotective Agents. J. Med. Chem. 2007, 50, 1731–1734.
- Couch, R. D.; Browning, R. G.; Honda, T.; Gribble, G. W.; Wright, D. L.; Sporn, M. B.; Anderson, A. C. Studies on the Reactivity of CDDO, a Promising New Chemopreventive and Chemotherapeutic Agent: Implications for a Molecular Mechanism of Action. Bioorg. Med. Chem. Lett. 2005, 15, 2215–2219.
- Honda, T.; Janosik, T.; Honda, Y.; Han, J.; Liby, K. T.; Williams, C. R.; Couch, R. D.; Anderson, A. C.; Sporn, M. B.; Gribble, G. W. Design, Synthesis, and Biological Evaluation of Biotin Conjugates of 2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic Acid for the Isolation of the Protein Targets. J. Med. Chem. 2004, 47, 4923–4932.
- Honda, T.; Favaloro, F. G., Jr.; Janosik, T.; Honda, Y.; Suh, N.; Sporn, M. B.; Gribble, G. W. Efficient Synthesis of (—)- and (+)-Tricyclic Compounds with Enone Functionalities in Rings A and C. A Novel Class of Orally Active Anti-inflammatory and Cancer Chemopreventive Agents. Org. Biomol. Chem. 2003, 1, 4384–4391.
- Favaloro, Jr., F. G.; Honda, T.; Honda, Y.; Gribble, G. W.; Suh, N.; Risingsong, R.; Sporn, M. B. Design and Synthesis of Tricyclic Compounds with Enone Functionalities in Rings A and C: A Novel Class of Highly Active Inhibitors of Nitric Oxide Production in Mouse Macrophages. J. Med. Chem. 2002, 45, 4801–4805.
- Honda, T.; Honda, Y.; Favaloro, Jr., F. G.; Gribble, G. W.; Suh, N.; Place, A. E.; Rendi, M. H.; Sporn, M. B. A Novel Dicyanotriterpenoid, 2-Cyano-3,12-dioxooloana-1,9(11)-dien-28-onitrile, Active at Picomolar Concentrations for Inhibition of Nitric Oxide Production. Bioorg. Med. Chem. Lett. 2002, 12, 1027–1030.
- Honda, T.; Rounds, B. V.; Bore, L.; Finlay, H. J.; Favaloro, Jr., F. G.; Suh, N.; Wang, Y.; Sporn, M. B.; Gribble, G. W. Synthetic Oleanane and Ursane Triterpenoids with Modified Rings A and C: A Series of Highly Active Inhibitors of Nitric Oxide Production in Mouse Macrophages. J. Med. Chem. 2000, 43, 4233–4246.
- Honda, T.; Gribble, G. W.; Suh, N.; Finlay, H. J.; Rounds, B. V.; Bore, L.; Favaloro, Jr., F. G.; Wang, Y.; Sporn, M. B. Novel Synthetic Oleanane and Ursane Triterpenoids with Various Enone Functionalities in Ring A as Inhibitors of Nitric Oxide Production in Mouse Macrophages. J. Med. Chem. 2000, 43, 1866–1877.
- Honda, T.; Rounds, B. V.; Gribble, G. W.; Suh, N.; Wang, Y.; Sporn, M. B. Design and Synthesis of 2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic Acid, a Novel and Highly Active Inhibitor of Nitric Oxide Production in Mouse Macrophages. Bioorg. Med. Chem. Lett. 1998, 8, 2711–2714.
- Honda, T.; Kato, M.; Inoue, M.; Shimamoto, T.; Shima, K.; Nakanishi, T.; Yoshida, T.; Noguchi, T. Synthesis and Antitumor Activity of Quaternary Ellipticine Glycosides, a Series of Novel and Highly Active Antitumor Agents. J. Med. Chem. 1988, 31, 1295–1305.