Faculty
Nancy S. Goroff, Professor Emeritus

B.A., 1990 Harvard University
Ph.D., 1994 UCLA
National Science Foundation Postdoctoral Fellow, Michigan State University, 1994-1996,
Michigan State University
Research Corporation Postdoctoral Fellow, University of Michigan 1996-1997
779 Chemistry
Email: nancy.goroff@stonybrook.edu
Awards/Honors
ACS Award for Creative Research and Applications of Iodine Chemistry, 2013
Award for Excellence in Service by a Graduate Program Director, Stony Brook Graduate
School, 2011
Journal Award, Synlett/Synthesis, Thieme Verlaag, 2003
National Science Foundation Career Award, 2000-2004
Organic Chemistry: Carbon-Rich Molecules and Materials
Our research probes the interface between organic chemistry and materials science, especially in the area of conjugated systems. Conjugated organic molecules and polymers have attracted increasing attention for applications as semi-conducting materials. Ideally, organic semiconductors are lighter in weight, more easily modifiable, and less expensive than their inorganic counterparts. These properties make highly unsaturated (carbon-rich) organics attractive, for example, as components in large-area displays, light-emitting diodes, chemical sensors, and even high-tech pigments. Work in the Goroff group focuses on finding new conjugated molecules and polymers with unusual electronic and optical properties, and using those materials to understand electronic and energy transport more thoroughly.
Halocarbon Chemistry for New Materials
One focus of our research is the chemistry of conjugated halocarbons, including iodoalkynes and halogenated cumulenes. We have prepared compounds 1-6 (Figure 1), most of which were previously unknown. The simplicity of these molecules conceals their interesting chemistry.
Compounds 1-3 contain strongly Lewis-acidic iodine atoms. We can use these acidic sites to guide self-assembly of macromolecular systems. We have used such ordered assemblies to polymerize 1 to poly(diiododiacetylene), or PIDA (7), a unique conjugated polymer consisting only of a linear carbon backbone and iodine-atom side groups. A major focus of our research is understanding the properties and chemistry of this new polymer. For example, we have found that treatment of PIDA suspensions with pyrollidine leads to a highly conductive new material, structure still unknown. We are also investigating the graphitization of PIDA fibers, which may take place under relatively mild conditions. In recent experiments, we have also obtained evidence for ordered polymerization of triyne 2 and tetrayne 3.
Recent experiments with PIDA have led to the discovery of a previously unreported reaction, namely the base-induced elimination of iodine from trans-diiodoalkenes to provide the corresponding alkynes (Figure 3). For PIDA, this reaction provides a potential route under clean, mild conditions to the linear carbon allotrope, carbyne.
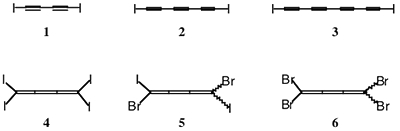
Figure 1. Halocarbon compounds 1-6 have all been prepared and studied in the Goroff lab.
 Figure 2. Topochemical polymerization of 1 gives poly(diiododiacetylene), or PIDA, 7.
Figure 2. Topochemical polymerization of 1 gives poly(diiododiacetylene), or PIDA, 7.
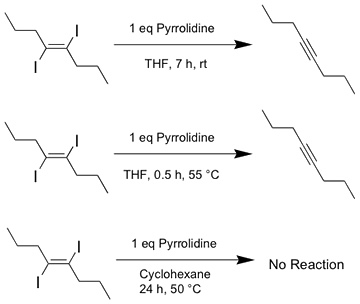
Figure 3. Topochemical polymerization of 1 gives poly(diiododiacetylene), or PIDA, 7
Conjugated Molecular Belts
Another major project in the Goroff lab centers on preparing tube-shaped cylindrically conjugated molecules. These compounds (e.g., 8, Figure 4) represent a presently unachieved class of organic materials for electronics applications.
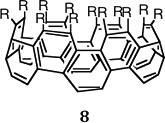 |
| Figure 4. A conjugated aromatic belt |
Similar to fullerenes and carbon nanotubes, these “buckybelts” will have a π system formed from adjacent benzene rings linked together into a cylinder. However, unlike fullerenes or nanotubes, the belts will have open edges which can be functionalized to alter the structures' physical, electronic, and chemical properties. In addition, the edges will allow for reversible binding of cations or small molecules inside the cylindrical cavity. This binding, which is impossible under ambient conditions for closed-cage fullerenes, make the belts potential components for nanoscale switches.
Our most recent approach to the buckybelts is to synthesize substituted cyclopentaphenanthrenes and attach them to a three-fold symmetric scaffold, such as in compound 9 (Figure 5). Coupling the carbonyl groups of 9 together by McMurry couplings will provide a direct precursor to the belt. Oxidative aromatization will complete the synthesis.
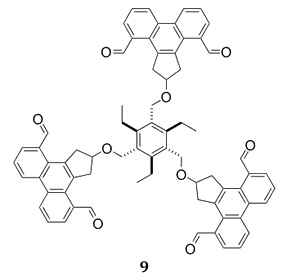
Figure 5. We are building compoung 9 as a precursor to the buckelt 8.
Publications
S. W. McElvany, M. M. Ross, N. S. Goroff, and F. Diederich, Science1993, 259, 1594-1596. Cyclocarbon coalescence: mechanism for tailor-made fullerene formation.
F. Diederich, Y. Rubin, O. L. Chapman, and N. S. Goroff, Helv. Chim. Acta1994, 77, 1441-1457. Synthetic routes to the cyclo[n]carbons.
N. S. Goroff, Acct. Chem. Res.1996, 29, 77-83. Mechanism of fullerene formation.
K. Gao and N. S. Goroff, J. Am. Chem. Soc.2000, 122, 9320-9321. Two new iodine-capped carbon rods.
P. D. Rege, O. L. Malkina, and N. S. Goroff, J. Am. Chem. Soc.2002, 124, 370-371. The effect of Lewis bases on the 13C NMR of iodoalkynes.
J. A. Webb, P.-H. Liu, O. L. Malkina, and N. S. Goroff, Angew. Chem., Int. Ed. 2002, 41, 3011-3014. Tetraiodobutatriene: A new cumulenic carbon iodide.
E. V. Dikarev, N. S. Goroff, and M. A. Petrukhina, J. Organomet. Chem., 2003, 683, 337-340. Expanding the scope of solvent-free synthesis: Entrapment of thermally unstable species.
J. A. Webb, J. E. Klijn, P. A. Hill, J. L. Bennett, and N. S. Goroff, J. Org. Chem., 2004, 69, 660-664. Experimental studies of the 13C NMR of iodoalkynes in Lewis basic solvents.
P.-H. Liu, L. Li, J. A. Webb, Y. Zhang, and N. S. Goroff, Org. Lett. 2004, 6, 2081-2083. Tetrabromobutatriene: Completing the perhalocumulene (C4X4) series.
W. N. Moss and N. S. Goroff, J. Org. Chem.2005,70, 802-808. Theoretical analysis of the 13C NMR of iodoalkynes upon complexation with Lewis bases.
N. S. Goroff, S. Curtis, J. A. Webb, J. W. Lauher, and F. W. Fowler, Org. Lett. 2005, 7, 1891-1893. Designed cocrystals based on the pyridine-iodoalkyne halogen bond.
A. Sun, J. W. Lauher, and N. S. Goroff, Science2006, 312, 1030-1034. Preparation of Poly(diiododiacetylene), an ordered conjugated polymer of carbon and iodine.
C. Wilhelm, S. A. Boyd, S. Chawda, F. W. Fowler, N. S. Goroff,* G. P. Halada, C. P. Grey, J. W. Lauher, L. Luo, C. D. Martin, J. B. Parise, C. Tarabrella, and J. A. Webb, J. Am. Chem. Soc. 2008, 130, 4415-4420. Pressure-induced polymerization of diiodobutadiyne in assembled co-crystals.
Luo, L.; Wilhelm, C.; Sun, A. W.; Grey, C. P.; Lauher, J. W.; Goroff, N. S. J. Am. Chem. Soc. 2008, 130, 7702. Poly(diiododiacetylene): Preparation, isolation, and full characterization of a very simple poly(diacetylene).
Lauher, J. W.; Fowler, F. W.; Goroff, N. S. Acc. Chem. Res. 2008, 41, 1215. Single-crystal-to-single-crystal topochemical polymerizations by design.
Luo, L.; Wilhelm, C.; Young, C. N.; Grey, C. P.; Halada, G. P.; Xiao, K.; Ivanov, I. N.; Howe, J. Y.; Geohegan, D. B.; Goroff, N. S. Macromolecules2011, 44, 2626. Characterization and carbonization of highly oriented poly(diiododiacetylene) nanofibers.
Luo, L.; Resch, D.; Wilhelm, C.; Young, C. N.; Halada, G. P.; Gambino, R. J.; Grey, C. P.; Goroff, N. S. J. Am. Chem. Soc.2011, 133, 19274. Room-temperature carbonization of poly(diiododiacetylene) by reaction with Lewis bases.
DeCicco, R. C.; Black, A.; Li, L.; Goroff, N. S. European Journal of Organic Chemistry2012, 4699. An iterative method for the synthesis of symmetric polyynes.
Jin, H.; Plonka, A. M.; Parise, J. B.; Goroff, N. S. Crystengcomm2013, 15, 3106. Pressure induced topochemical polymerization of diiodobutadiyne: a single-crystal-to-single-crystal transformation.