Nanomaterials for Fuel Cells
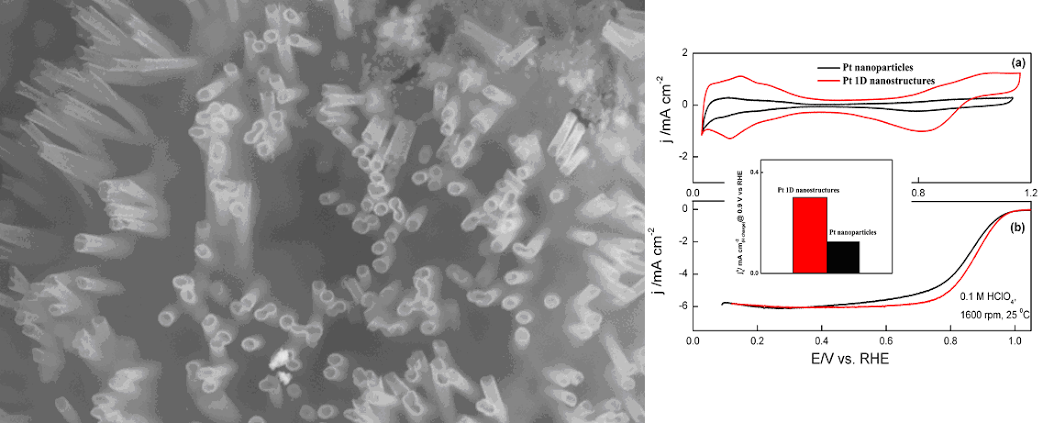
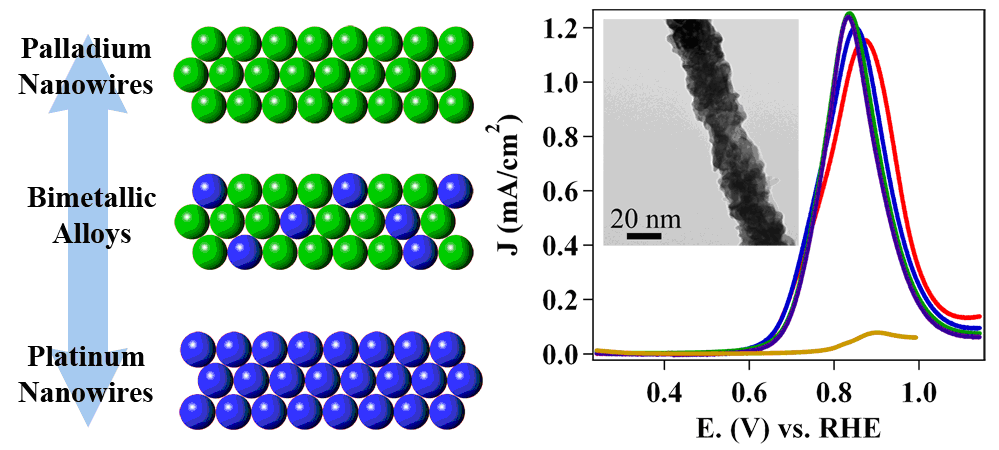
1. One-dimensional (1-D) metal (Ag, Au and Pt) nanowires and their corresponding arrays have been synthesized using an ambient, surfactantless synthesis technique. The potential applicability of such crystalline, highly purified 1-D samples for practical uses was specifically demonstrated in their manifestation as electrocatalysts for an oxygen reduction reaction (ORR). Specifically, Pt 1-D nanostructures possessed a two-fold higher ORR activity as compared with that of commercial Pt nanoparticles alone. Ag and Au nanowires also evinced reasonable ORR activity in alkaline solution. Ref.: J. Phys. Chem. C, v.113, 5460 (2009).
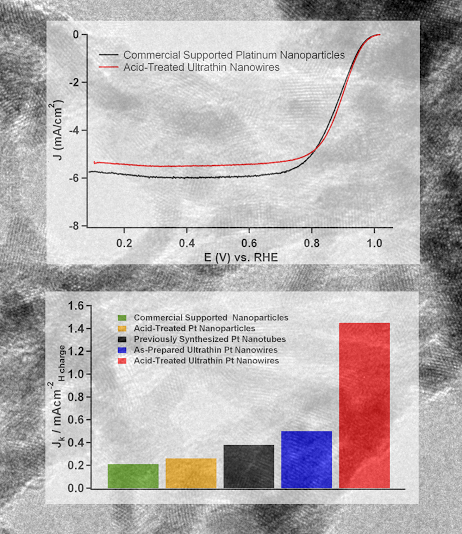
2. We report on the synthesis, characterization, and electrocatalytic performance of ultrathin Pt nanowires with a diameter of less than 2 nm. An acid-wash protocol was employed in order to yield highly exfoliated, crystalline nanowires with a diameter of 1.3 ± 0.4 nm. The electrocatalytic activity of these nanowires toward the oxygen reduction reaction was studied in relation to the activity of both supported and unsupported Pt nanoparticles as well as with previously synthesized Pt nanotubes. Our ultrathin, acid-treated, unsupported nanowires displayed an electrochemical surface area activity of 1.45 mA/cm2, which was nearly 4 times greater than that of analogous, unsupported platinum nanotubes and 7 times greater than that of commercial supported platinum nanoparticles. Ref.: Nano Lett., v.10, 2806 (2010).
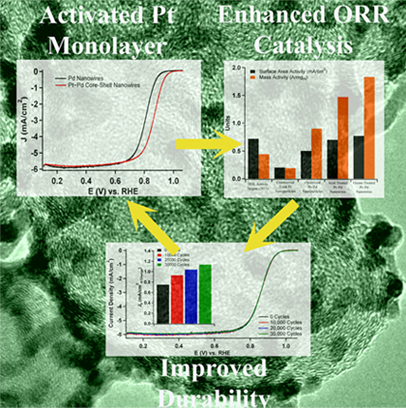
3. We report on the synthesis, characterization, and electrochemical performance of novel,ultrathin Pt monolayer shell–Pd nanowire core catalysts. Initially, ultrathin Pd nanowires with diameters of 2.0 ± 0.5 nm were generated, and a method has been developed to achieve highly uniform distributions of these catalysts onto the Vulcan XC-72 carbon support. As-prepared wires are activated by the use of two distinctive treatment protocols followed by selective CO adsorption in order to selectively remove undesirable organic residues. Subsequently, the desired nanowire core-Pt monolayer shell motif was reliably achieved by Cu underpotential deposition followed by galvanic displacement of the Cu adatoms. The surface area and mass activity of the acid and ozone-treated nanowires were assessed, and the ozone-treated nanowires were found to maintain outstanding area and mass specific activities of 0.77 mA/cm2 and 1.83 A/mgPt, respectively, which were significantly enhanced as compared with conventional commercial Pt nanoparticles, core-shell nanoparticles, and acid-treated nanowires. The ozone-treated nanowires also maintained excellent electrochemical durability under accelerated half-cell testing, and it was found that the area-specific activity increased by 1.5 fold after a simulated catalyst lifetime. Ref.: J. Am. Chem. Soc., v.133, 9783 (2011)
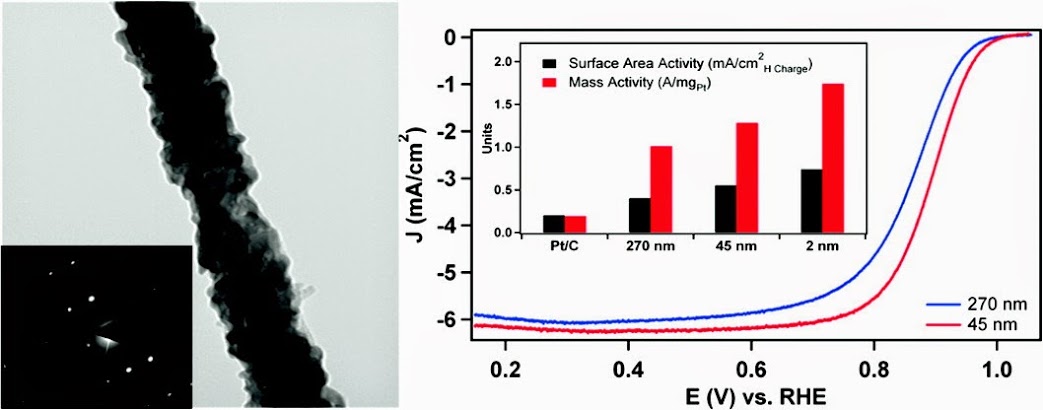
4. In this report, we utilize the U-tube double diffusion device as a reliable, environmentally friendly method for the size-controlled synthesis of high-quality, single crystalline Pd nanowires. The nanowires grown in 200 and 15 nm polycarbonate template pores maintain diameters of 270 ± 45 nm and 45 ± 9 nm, respectively, and could be isolated either as individual nanowires or as ordered free-standing arrays. The growth mechanism of these nanowires has been extensively explored, and we have carried out characterization of the isolated nanowires, free-standing nanowire arrays, and cross sections of the filled template in order to determine that a unique two-step growth process predominates within the template pores. Moreover, as-prepared submicrometer and nanosized wires were studied by comparison with ultrathin 2 nm Pd nanowires in order to elucidate the size-dependent trend in oxygen reduction reaction (ORR) electrocatalysis. Subsequently, the desired platinum monolayer overcoating was reliably deposited onto the surface of the Pd nanowires by Cu underpotential deposition (UPD) followed by galvanic displacement of the Cu adatoms. The specific and platinum mass activity of the core-shell catalysts was found to increase from 0.40 mA/cm2 and 1.01 A/mg to 0.74 mA/cm2 and 1.74 A/mg as the diameter was decreased from the submicrometer size regime to the ultrathin nanometer range. Ref.: ACS Nano., v.5(9), 7471-7487 (2011).
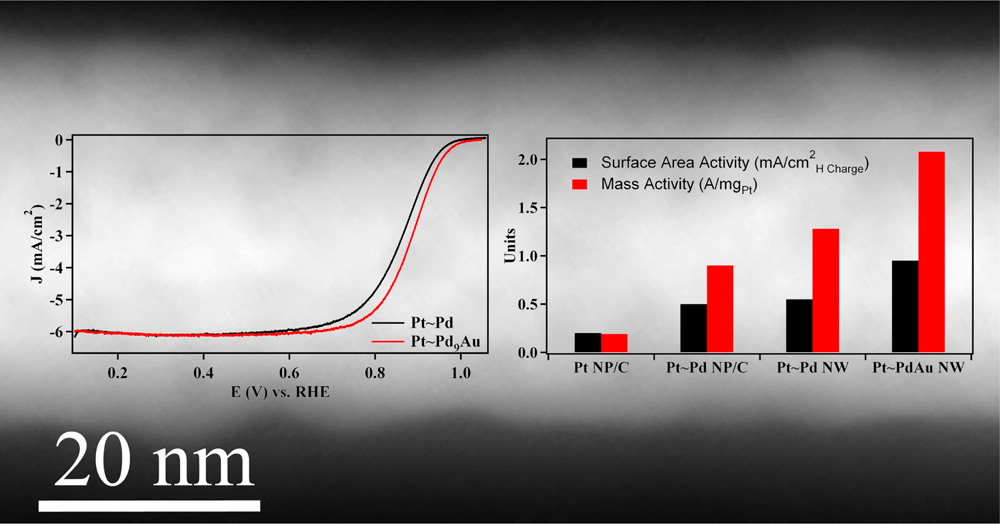
5. We have employed an ambient, template-based technique that is simple, efficient, and surfactantless to generate a series of bimetallic Pd1-xAux and Pd1-xPtx nanowires with control over composition and size. Our as-prepared nanowires maintain significantly enhanced activity toward oxygen reduction as compared with commercial Pt nanoparticles and other 1D nanostructures, as a result of their homogeneous alloyed structure. Specifically, Pd9Au and Pd4Pt nanowires possess oxygen reduction reaction (ORR) activities of 0.49 and 0.79 mA/cm2, respectively, which are larger than the analogous value for commercial Pt nanoparticles (0.21 mA/cm2). In addition, core-shell Pt~Pd9Au nanowires have been prepared by electrodepositing a Pt monolayer shell and the corresponding specific, platinum mass, and platinum group metal mass activities were found to be 0.95 mA/cm2, 2.08 A/mgPt and 0.16 A/mgPGM, respectively. The increased activity and catalytic performance is accompanied by improved durability toward ORR. Ref.: Nano Lett., v.12(4), 2013-2020 (2012).
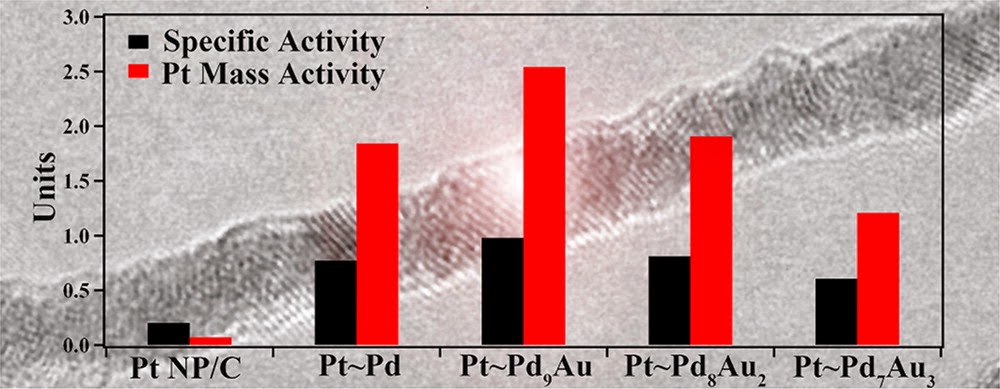
6. In this report, we examine the composition- and size-dependent performance in hierarchical Pd1-xAux nanowires (NWs) encapsulated with a conformal Pt monolayer shell (Pt~Pd1-xAux). The ultrathin Pd1-xAux NWs are prepared by a solution-based method wherein the chemical composition can be readily and predictably controlled. Importantly, as-prepared Pd9Au NWs maintain significantly enhanced oxygen reduction reaction (ORR) activity (0.40 mA/cm2), as compared with elemental Pd NW/C (0.12 mA/cm2) and Pt nanoparticles (NP)/C (0.20 mA/cm2), respectively. After the deposition of a Pt monolayer, a volcano-type composition dependence is observed in the ORR activity of the Pt~Pd1-xAux NWs as the Au content is increased from 0 to 30% with the activity of the Pt~Pd9Au NWs (0.98 mA/cm2, 2.54 A/mgPt), representing the optimum performance. We note that the platinum group metal activity of the ultrathin 2 nm NWs (0.64 A/mg) is significantly enhanced as compared with that of analogous 50 nm NWs (0.16 A/mg) and commercial Pt NP/C (0.1-0.2 A/mg), thereby highlighting a distinctive size-dependent enhancement in NW performance. Ref.: J. Phys. Chem. C, v.116(29), 15297-15306 (2012).
7. This Perspective discusses how despite increasing interest in the use of one-dimensional (1D) noble metal nanostructures for the oxygen reduction reaction, there has been a surprising lack of effort expended in thoroughly and rationally examining the influence of various physicochemical properties of 1D electrocatalysts with respect to their intrinsic performance. In this Perspective, we address this important issue by investigating and summarizing recent theoretical and experimental progress aimed at precisely deducing the nature of the complex interplay among size, chemical composition, and electrocatalytic performance in high-quality elemental and bimetallic 1D noble metal nanowire systems. In terms of these structural parameters, significant enhancements in both activity and durability of up to an order of magnitude in the case of Pt-Pd1-xAux nanowires, for example, can be achieved by rationally tuning both wire size and composition. The fundamental insights acquired are then utilized to discuss future and potentially radically new directions toward the continuous improvement and optimization of 1D catalysts. Ref.: J. Phys. Chem. Lett. (cover), v.3(22), 3385-3398 (2012).
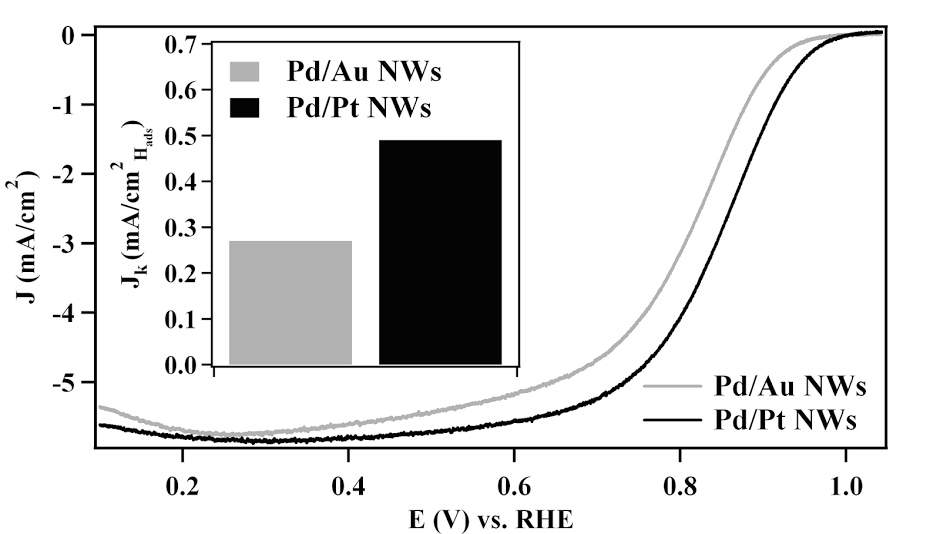
8. Segmented noble metal nanowires (NWs) represent an exciting, multifunctional one-dimensional structural architecture with a variety of potential applications. However, the wide-spread use of electrodeposition in the preparation of these systems has limited their potential to be produced on a large scale, since this protocol is costly and requires complex processes and caustic reaction media. Given the inherent limitations of electrodeposition, we report for the first time an ambient, surfactantless template-based approach, that is not only sustainable but also efficient for the reliable production of Pd/Pt and Pd/Au segmented NWs, possessing two spatially separated, chemically distinctive but elementally pure, axial subunits. Our simple two-step synthetic approach allows for direct and predictable control over the relative segment lengths in these nanomaterials. Moreover, thorough structural characterization of these as-prepared samples confirms that our segmented NWs maintain high quality, crystalline, elementally pure sub-units with a well-defined interface between the constituent metals. In the context of preparing segmented NWs as multifunctional nanostructures, we demonstrate that these as-prepared NWs achieve high levels of performance when employed as both electrocatalysts and nanomotors. Ref.: invited contribution, ‘Nanochemistry’ special issue, Israel Journal of Chemistry, v.52(11-12), 1090 (2012).
9. We report for the first time (a) the synthesis of elemental Ru NWs, (b) a method for modifying their surfaces with Pt, and (c) the morphology-dependent methanol oxidation reaction (MOR) performance of high-quality Pt-modified Ru NW electrocatalysts. The synthesis of our elemental Ru NWs has been accomplished, utilizing a template-based method under ambient conditions. As-prepared Ru NWs are crystalline and elementally pure, maintain electrochemical properties analogous to elemental Ru, and can be generated with average diameters ranging from 50 to 280 nm. We rationally examine the morphology-dependent performance of the Ru NWs by comparison with commercial Ru NP/C after decorating the surfaces of these structures with Pt. We have demonstrated that the deposition of Pt onto the Ru NWs (Pt~Ru NWs) results in a unique hierarchical structure, wherein the deposited Pt exists as discrete clusters on the surface. By contrast, we find that the Pt-decorated commercial Ru NP/C (Pt~Ru NP/C) results in the formation of an alloy-type nanoparticle. The Pt~Ru NPs (0.61 A/mgPt) possess nearly two-fold higher specific activity than analogous Pt~Ru NW electrocatalysts (0.36 A/mgPt). On the basis of a long term durability test, it is apparent that both catalysts undergo significant declines in performance, potentially resulting from aggregation and ripening in the case of the Pt~Ru NP/C and the effects of catalyst poisoning in the Pt~Ru NWs. At the conclusion of the test, both catalysts maintain comparable performance, despite a slightly enhanced performance in the Pt~Ru NP/C. In addition, the measured mass-normalized MOR activity of the Pt~Ru NWs (0.36 A/mgPt) was significantly enhanced as compared with carbon-supported elemental Pt (Pt NP/C, 0.09 A/mgPt) and alloy-type PtRu (PtRu NP/C, 0.24 A/mgPt) nanoparticles, both serving as commercial standards. Ref.: ACS Appl. Mater. Interf., v.5(12), 5518-5530 (2013).
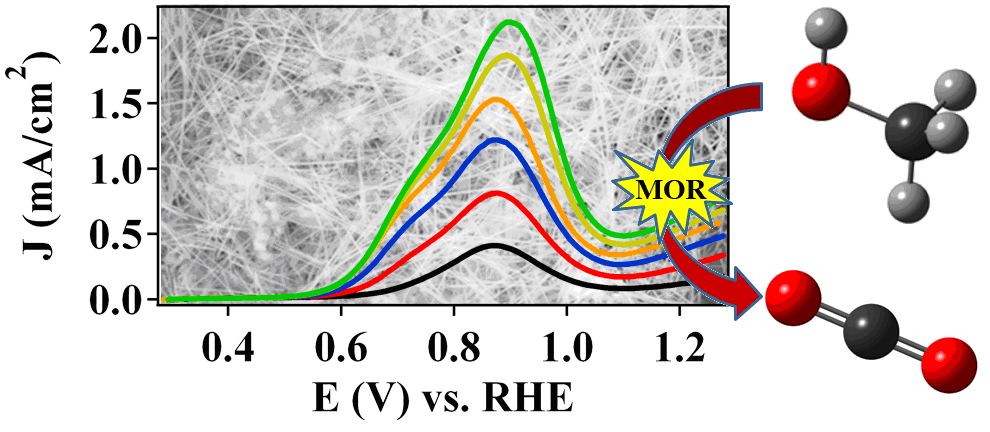
10. In this report, we address two key challenges in the development of electrocatalysts for direct methanol fuel cells by rationally tailoring the morphology and chemical composition of Pd-based nanowires (NWs) for enhanced performance. First, we have examined the morphology and composition-dependent performance of Pt1-xPdx NWs towards the methanol oxidation reaction (MOR). Elemental Pt NWs were found to possess a significant morphology-dependent enhancement of nearly three-fold in terms of peak MOR specific activity over that of commercial Pt NP/C. In addition, tailoring the chemical composition in Pt1-xPdx NWs can lead to measurable increases in MOR kinetics, which can be attributed to improved oxidation of formic acid and potentially, increased selectivity for a direct, CO-free pathway. Second, we have explored the stability of ORR performance in the presence of measurable concentrations of methanol as a function of chemical composition in Pt1-xPdx NWs and Pt-free Pd9Au NWs. In the context of the Pt1-xPdx NWs, a distinctive volcano-type dependence has been noted with respect to chemical composition, and on the basis of the MOR activities and methanol tolerant ORR behavior, Pt7Pd3 NWs have been highlighted as an optimal catalyst architecture. We have also analyzed the methanol tolerance in Pd9Au NWs, which represent a highly active, durable Pt-free alternative to traditional Pt-based nanostructured catalysts. Herein, we have demonstrated that Pd9Au NWs (0.42 mA/cm2) with no effective Pt content can outperform Pt-based nanostructures, such as Pt NWs (0.32 mA/cm2) and nanoparticulate Pt NP/C (0.24 mA/cm2) in the presence of 4 mM methanol/0.1 M HClO4. Ref.: ACS Catalysis, v.3(9), 2031-2040 (2013).
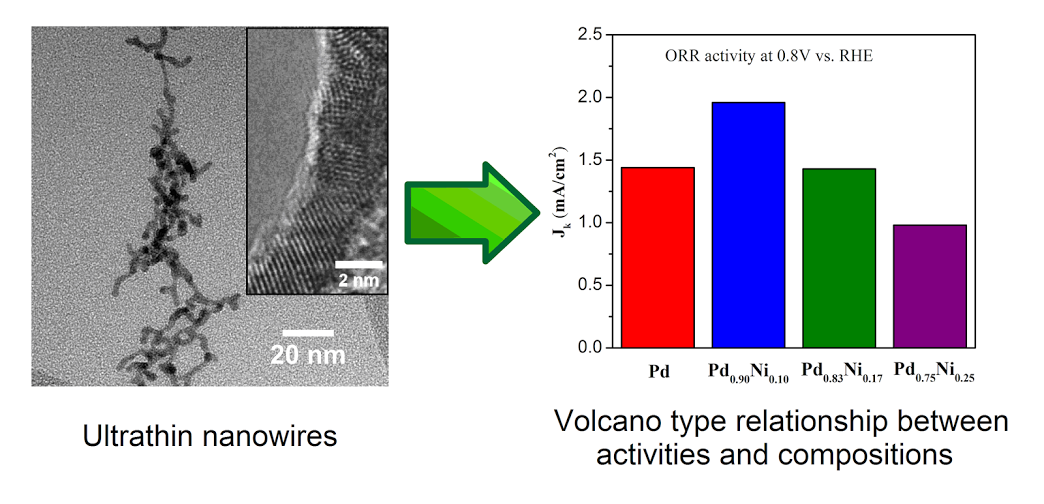
11. An ambient, surfactant-based synthetic means was used to prepare ultrathin binary (‘d’ ~ 2 nm) Pd-Ni nanowires, which were subsequently purified using a novel butylamine-based surfactant exchange process coupled with an electrochemical CO stripping treatment in order to expose active surface sites. We were able to systematically vary the chemical composition of as-prepared Pd-Ni nanowires from pure elemental Pd to Pd0.50Ni0.50 (atomic ratio), as verified using EDS analysis. The overall morphology of samples possessing greater than 60 atom % Pd consisted of individual, discrete one-dimensional nanowires. The electrocatalytic performances of elemental Pd, Pd0.90Ni0.10, Pd0.83Ni0.17, and Pd0.75Ni0.25nanowires in particular were examined. Our results highlight a “volcano”-type relationship between chemical composition and corresponding ORR activities with Pd0.90Ni0.10 yielding the highest activity (i.e. 1.96 mA/cm2 at 0.8 V) amongst all nanowires tested. Moreover, the Pd0.90Ni0.10 sample exhibited outstanding methanol tolerance ability. In essence, there was only a relatively minimal 15% loss in the specific activity in the presence of 4 mM methanol, which was significantly better than analogous data on Pt nanoparticles and Pt nanowires. In addition, we also studied ultrathin, core-shell Pt~Pd0.90Ni0.10 nanowires, which exhibited a specific activity of 0.62 mA/cm2 and a corresponding mass activity of 1.44 A/mgPt at 0.9 V. Moreover, our as-prepared core-shell electrocatalysts maintained excellent electrochemical durability. We postulate that one-dimensional Pd-Ni nanostructures represent a particularly promising platform for designing ORR catalysts with high performance. Ref.: ACS Catalysis, v.4(8), 2544-2555 (2014).
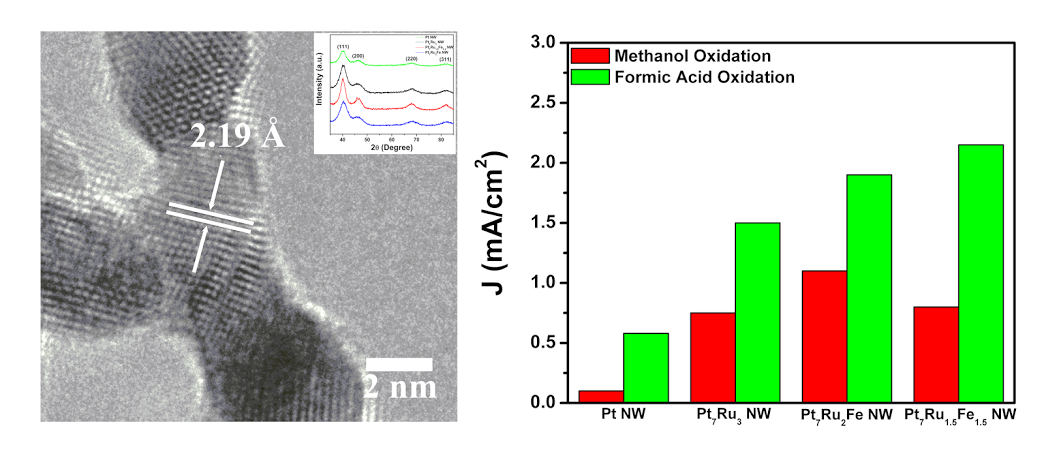
12. In the search for alternatives to conventional Pt electrocatalysts, we have synthesizedultrathin, ternary PtRuFe nanowires (NW), possessing different chemical compositions in order to probe their CO tolerance as well as electrochemical activity as a function of composition for both (i) the methanol oxidation reaction (MOR) and (ii) the formic acid oxidation reaction (FAOR). As-prepared ‘multifunctional’ ternary NW catalysts exhibited both higher MOR and FAOR activity as compared with binary Pt7Ru3 NW controls, mono-metallic Pt NWs, and commercial catalyst samples. In terms of synthetic novelty, we utilized a sustainably mild, ambient wet-synthesis method never previously applied to the fabrication of crystalline, pure ternary systems in order to fabricate ultrathin, homogeneous alloy PtRuFe NWs with a range of controlled compositions. These NWs were subsequently characterized using a suite of techniques including XRD, TEM, SAED, and EDAX in order to verify not only the incorporation of Ru and Fe into the Pt lattice but also their chemical homogeneity, morphology, as well as physical structure and integrity. Lastly, these NWs were electrochemically tested in order to deduce the appropriateness of conventional explanations such as (i) the bi-functional mechanism as well as (ii) the ligand effect to account for our MOR and FAOR reaction data. Specifically, methanol oxidation appears to be predominantly influenced by the Ru content, whereas formic acid oxidation is primarily impacted by the corresponding Fe content within the ternary metal alloy catalyst itself. Ref.: Energy & Environmental Sciences, v.8(1), 350-363 (2015).
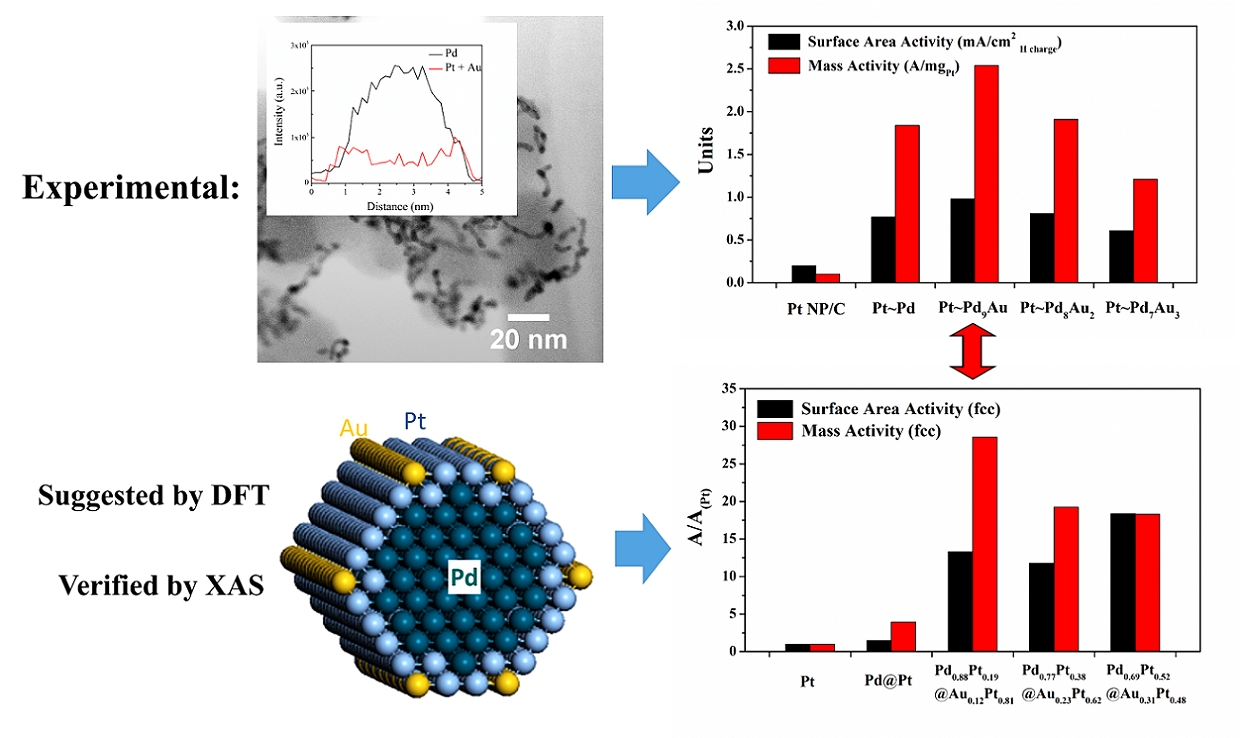
13. To create truly effective electrocatalysts for the cathodic reaction governing proton exchange membrane fuel cells (PEMFC), namely the oxygen reduction reaction (ORR), necessitates an accurate and detailed structural understanding of these electrocatalysts, especially at the nanoscale, and to precisely correlate that structure with demonstrable performance enhancement. To address this key issue, we have combined and interwoven theoretical calculations with experimental, spectroscopic observations in order to acquire useful structural insights into the active site geometry with implications for designing optimized nanoscale electrocatalysts with rationally predicted properties. Specifically, we have probed ultrathin (~2 nm) core-shell Pt~Pd9Au nanowires, which has been previously shown to be excellent candidates for ORR in terms of both activity and long-term stability, from the complementary perspectives of both DFT calculations and X-ray Absorption Spectroscopy (XAS). The combination and correlation of data from both experimental and theoretical studies has revealed for the first time that the catalytically active structure of our ternary nanowires can actually be ascribed to PtAu~Pd possessing a PtAu binary shell and a pure inner Pd core. Moreover, we have plausibly attributed the resulting structure to a specific synthesis step, namely the Cu underpotential deposition (UPD) followed by galvanic replacement with Pt. Hence, the fundamental insights gained into the performance of our ultrathin nanowires from our demonstrated approach will likely guide future directed efforts aimed at broadly improving upon the durability and stability of nanoscale electrocatalysts in general. Ref.:J. Am. Chem. Soc., v.137(39), 12597-12609 (2015).
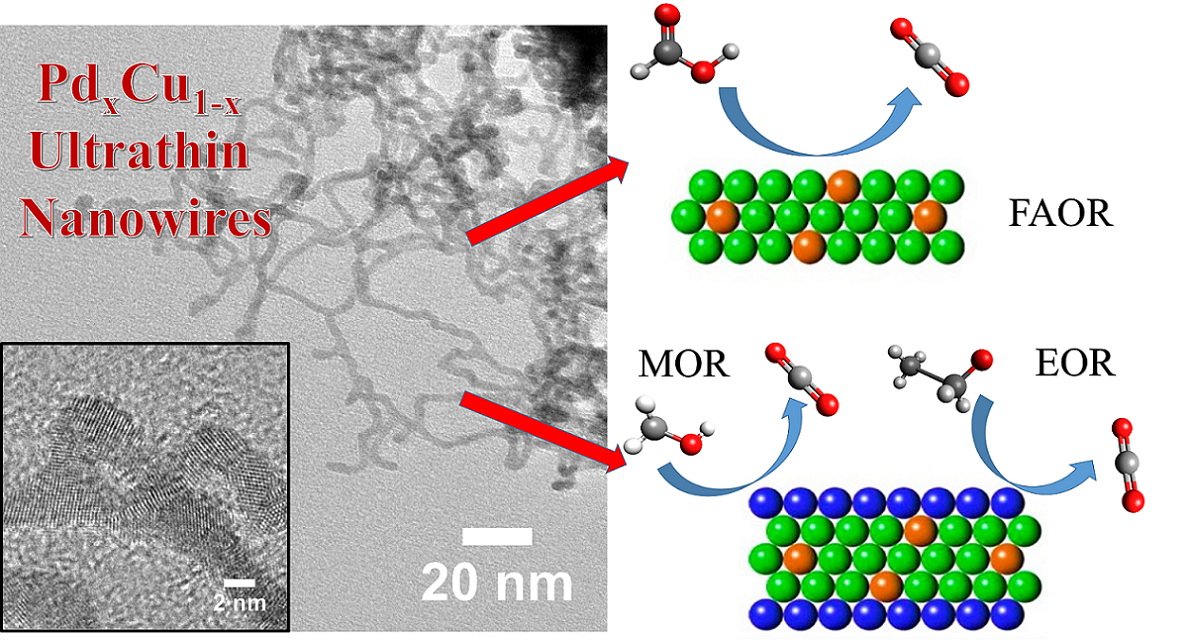
14. Developing novel electrocatalysts for small molecule oxidation processes, including formic acid oxidation (FAOR), methanol oxidation reaction (MOR), and ethanol oxidation reaction (EOR), denoting the key anodic reactions for their respective fuel cell configurations, is a significant and relevant theme of recent efforts in the field. Herein in this report, we demonstrated a concerted effort to couple and combine the benefits of small size, anisotropic morphology, and tunable chemical composition in order to devise a novel ‘family’ of functional architectures. In particular, we have fabricated not only ultrathin 1-D Pd1-xCux alloys but also Pt-coated Pd1-xCux(i.e. Pt~Pd1-xCux) core-shell hierarchical nanostructures with readily tunable chemical compositions by utilizing a facile, surfactant-based, wet chemical synthesis coupled with a Cu underpotential deposition technique. Our main finding is that our series of as-prepared nanowires are functionally flexible. More precisely, we demonstrate that various examples within this ‘family’ of structural motifs can be tailored for exceptional activity with all 3 of these important electrocatalytic reactions. In particular, we note that our series of Pd1-xCux nanowires all exhibit enhanced FAOR activities as compared with not only analogous Pd ultrathin nanowires but also commercial Pt and Pd standards, with Pd9Cu representing the “optimal” composition. Moreover, our group of Pt~Pd1-xCux nanowires consistently outperformed not only commercial Pt NPs but also ultrathin Pt nanowires by several fold orders of magnitude for both the MOR and EOR reactions in alkaline media. The variation of the MOR and EOR performance with the chemical composition of our ultrathin Pt~Pd1-xCux nanowires was also discussed. Ref.: ACS Appl. Mater. Interf., v.7(47), 26145–26157 (2015).
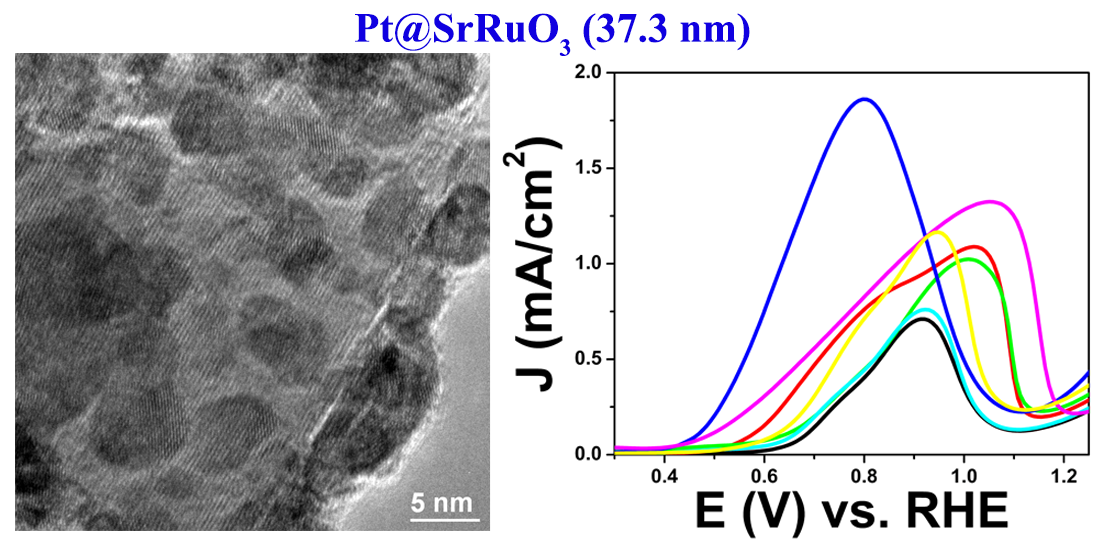
15. The performance of electrode materials in conventional direct alcohol fuel cells (DAFC) is constrained by (i) the low activity of the catalyst materials relative to their overall cost, (ii) the poisoning of the active sites due to the presence of carbon monoxide produced during small molecule oxidation, and (iii) the lack of catalytic stability and durability on the underlying commercial carbon support. Therefore, as a viable alternative, we have synthesized various metal oxide and perovskite materials of different sizes and chemical compositions as supports for Pt nanoparticles (NPs). Our results including unique mechanistic studies demonstrate that the SrRuO3 substrate with immobilized Pt NPs at its surface evinces the best methanol oxidation performance as compared with all of the other substrate materials tested, including commercial carbon itself. Additionally, data from electron energy loss spectroscopy (EELS) and X-ray photoelectron spectroscopy (XPS) confirmed the presence of electron transfer from bound Pt NPs to surface Ru species within the SrRuO3 substrate itself, thereby suggesting that favorable metal-support interactions are responsible for the increased MOR activity of Pt species with respect to the underlying SrRuO3 composite catalyst material. Ref.: Catalysis Science and Technology, v.6(7), 2435-2450 (2016).
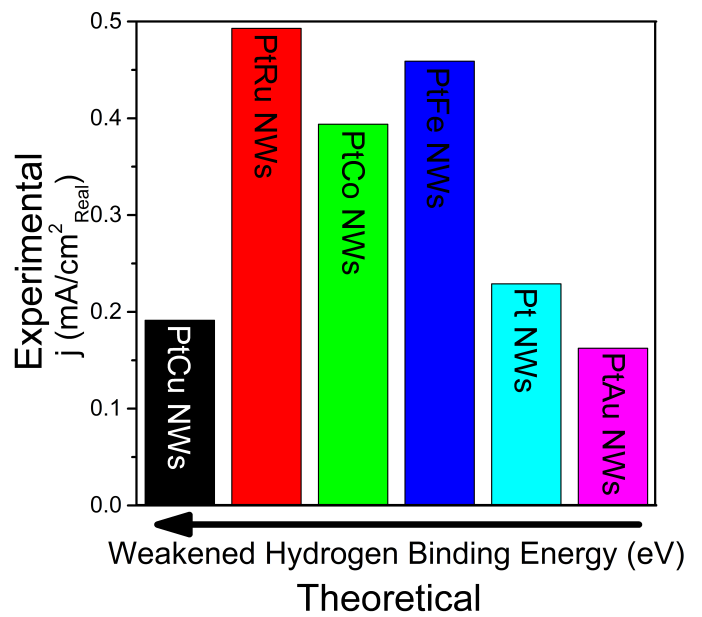
16. With an increased interest in the development of hydrogen fuel cells as a plausible alternative to combustion engines, recent work has focused on creating alkaline fuel cells (AFC), which employ an alkaline environment. Working in alkaline as opposed to acidic media yields a number of tangible benefits, including (i) the ability to use cheaper and plentiful precious-metal-free catalysts, due to their increased stability; (ii) a reduction in the amount of degradation and corrosion of Pt-based catalysts; and (iii) a longer operational lifetime for the overall fuel cell configuration. However, in the absence of Pt, no catalyst has achieved similar activities to that of Pt. Herein, we have synthesized a number of crystalline ultrathin PtM alloy nanowires (NWs) (‘M’ = Fe, Co, Ru, Cu, and Au)in order to replace a portion of the costly Pt metal without compromising on activity while simultaneously adding in metals known to exhibit favorable synergistic ligand and strain effects with respect to the host lattice. In fact, our experiments confirm theoretical insights about a clear and correlative dependence between measured activity and chemical composition. We have conclusively demonstrated that our as-synthesized alloy NW catalysts yield improved hydrogen oxidation reaction (HOR) activities as compared with a commercial Pt standard as well as with our as-synthesized Pt NWs. The Pt7Ru3 NW system, in particular, quantitatively achieved an exchange current density of 0.493 mA/cm2, which is higher than the corresponding data for Pt NWs alone. Additionally, the HOR activities follow the same expected trend as their calculated hydrogen binding energy (HBE) values, thereby confirming the critical importance and correlation of HBE with the observed activities. Ref.: ACS Catalysis, v.6(6), 3895-3908 (2016).
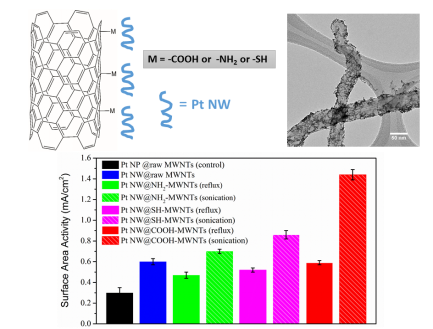
17. Multi-walled carbon nanotubes (MWNTs) represent a promising support medium for electrocatalysts, especially Pt nanoparticles (NPs). The advantages of using MWNTs include their large surface area, high conductivity, as well as long-term stability. Surface functionalization of MWNTs with various terminal groups such as -COOH, -SH, and -NH2 allows for rational electronic tuning of catalyst-support interactions. However, several issues still need to be addressed for such systems. First, over the course of an electrochemical run, catalyst durability can decrease, due in part to metal NP dissolution, a process facilitated by the inherently high surface defect concentration within the support. Second, the covalent functionalization treatment of MWNTs adopted by most groups tends to lead to a loss of structural integrity of the nanotubes (NTs). To mitigate for all of these issues, we have utilized two different attachment approaches (i.e. covalent versus non-covalent) to functionalize the outer walls of pristine MWNTs and compared the catalytic performance of as-deposited ultrathin (< 2 nm) 1D Pt nanowires with that of conventional Pt NPs towards the oxygen reduction reaction (ORR). Our results demonstrated that the electrochemical activity of Pt nanostructures immobilized onto functionalized carbon nanotube (CNT) supports could be dramatically improved by using ultrathin Pt nanowires (instead of NPs) with non-covalently (as opposed to covalently) functionalized CNT supports. Spectroscopic evidence corroborated the definitive presence of charge transfer between the metal catalysts and the underlying NT support, whose direction and magnitude are a direct function of (i) the terminal chemistry as well as (ii) the attachment methodology, all of which simultaneously impact upon the observed electrocatalytic performance. Specifically, the use of a non-covalent π-π stacking method coupled with a -COOH terminal moiety yielded the highest performance results, reported to date, for any similar system consisting of Pt (commercial NPs or otherwise) deposited onto carbon-based supports, a finding of broader interest towards the fabrication of high-performing electrocatalysts in general. Ref.: ACS Appl. Mater. Interf., v.8(50), 34280–34294 (2016).
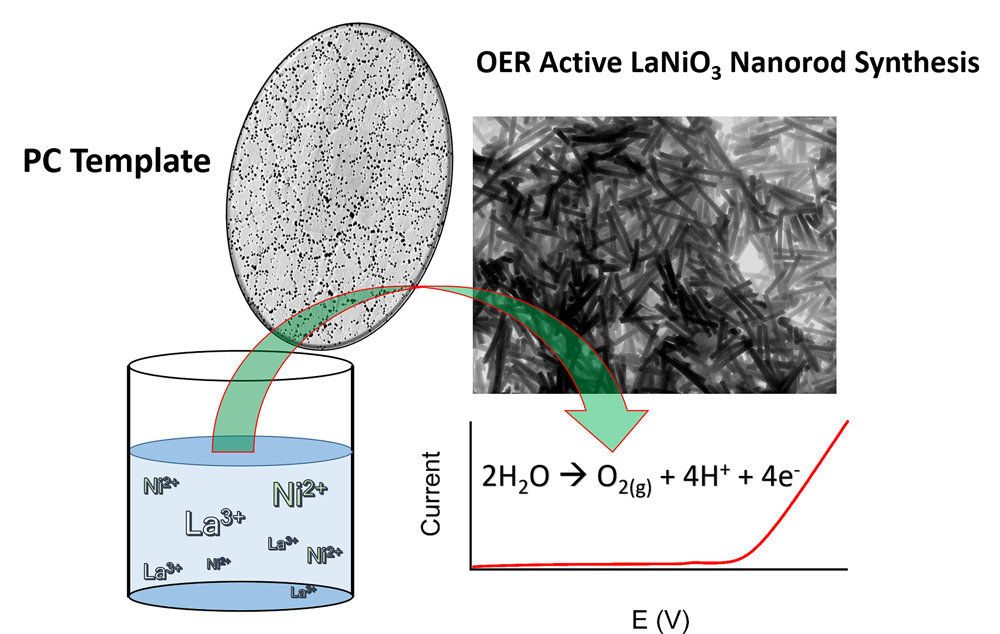
18. The oxygen evolution reaction (OER) is a key reaction for water electrolysis cells and air-powered battery applications. However, conventional metal oxide catalysts, used for high-performing OER, tend to incorporate comparatively expensive and less abundant precious metals such as Ru and Ir, and moreover, suffer from poor stability. To mitigate for all of these issues, we have prepared one-dimensional OER-active perovskite nanorods using a unique, simple, generalizable, and robust method. Significantly, our work demonstrates the feasibility of a novel electroless, seedless, surfactant-free, wet solution-based protocol for fabricating ‘high aspect ratio’ LaNiO3 and LaMnO3nanostructures. As the main focus of our demonstration of principle, we prepared as-synthesized LaNiO3 rods and correlated the various temperatures at which these materials were annealed with their resulting OER performance. We observed generally better OER performance for samples prepared with lower annealing temperatures. Specifically, when annealed at 600ºC, in the absence of a conventional conductive carbon support, our as-synthesized LaNiO3 rods not only evinced (i) a reasonable level of activity towards OER but also displayed (ii) an improved stability, as noted from chronoamperometric measurements, especially when compared with a control sample of commercially available (and more expensive) RuO2. Ref.:ACS Applied Materials and Interfaces, v.9, 24634 (2017).
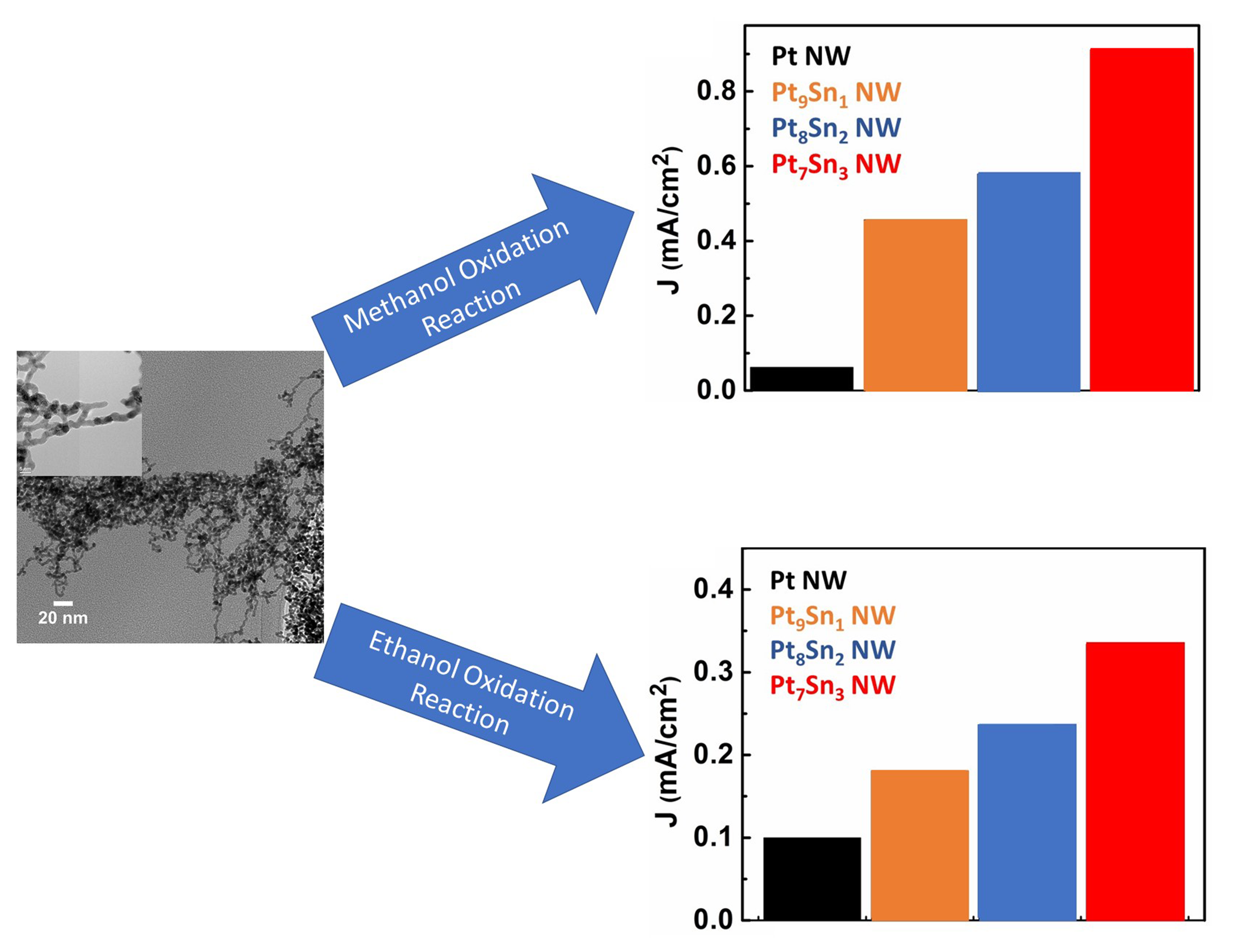
19. Pt-based alloys denote promising catalysts for methanol oxidation reaction (MOR) and ethanol oxidation reaction (EOR), due to their enhanced activity towards alcohol-oxidation reactions and reduced cost as compared with Pt alone. Among all of these binary systems, PtSn has been reported to exhibit superior methanol/ethanol oxidation activity. In this study, we deliberatively tailor chemical composition, reduce size, and optimize morphology of the catalyst in an effort to understand structure-property correlations that can be used to improve upon the electro-catalytic activity of these systems. Previous work performed by our group suggested that Pt-based catalysts, possessing an ultrathin one-dimensional (1D) structure, dramatically promote both cathodic and anodic reactions with respect to their zero-dimensional (0D) counterparts. Herein, a novel set of ultrathin binary Pt-Sn 1D nanowire (NW) catalysts with rationally controlled chemical compositions, i.e. Pt9Sn1, Pt8Sn2, and Pt7Sn3, have been synthesized using a facile, room-temperature, wet-solution-based method. The crystallinity and chemical composition of these as-prepared samples were initially characterized using XRD, XPS, and EDX. Results revealed that this synthetic protocol could successfully generate PtSn alloys with purposely tunable chemical compositions. TEM and HRTEM verified the structural integrity of our ultrathin 1D NW morphology for our Pt9Sn1, Pt8Sn2, and Pt7Sn3 samples. The effect of varying Sn content within these alloy samples towards the electro-oxidation reaction of methanol and ethanol were probed using cyclic voltammetry (CV) in acidic media. Within this series, we find that the optimized chemical composition for both the methanol oxidation reaction (MOR) and the ethanol oxidation reaction (EOR) is Pt7Sn3. Ref.: invited article, ACS Applied Nano Materials, 1(3), 1104–1115 (2018).
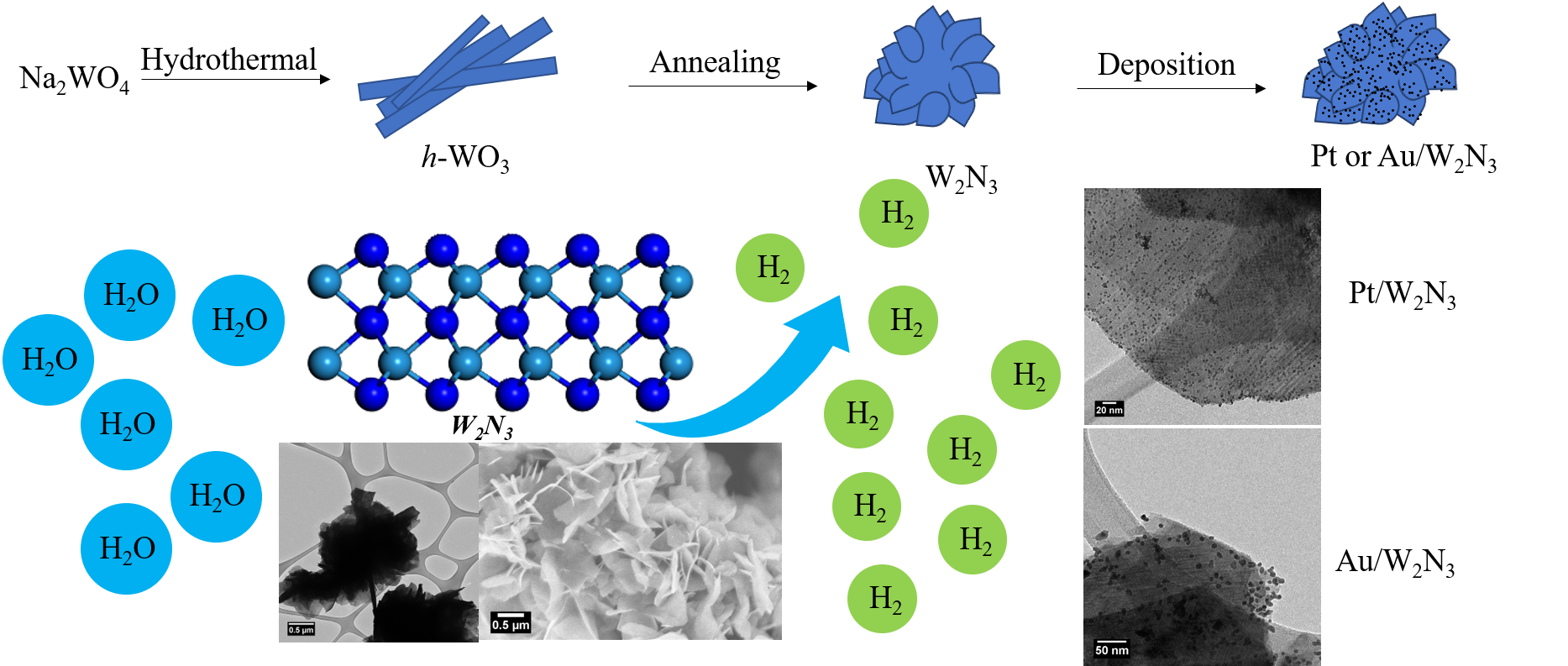
20. We have sought to improve the electrocatalytic performance of tungsten nitride through synthetic control over chemical composition and morphology. In particular, we have generated a thermodynamically unstable but catalytically promising nitrogen-rich phase of tungsten via a hydrothermal generation of a tungsten oxide intermediate and subsequent annealing in ammonia. The net product consisted of three-dimensional (3D) micron-scale flower-like motifs of W2N3; this architecture not only evinced high structural stability but also incorporated the favorable properties of constituent two-dimensional nanosheets. From a performance perspective, as-prepared 3D W2N3 demonstrated promising hydrogen evolution reaction (HER) activities, especially in an acidic environment with a measured overpotential value of -101 mV at a current density of 10 mA/cm2. To further enhance the electrocatalytic activity, small amounts of precious metal nanoparticles (such as Pt and Au), consisting of variable sizes, were uniformly deposited onto the underlying 3D W2N3 motifs using a facile direct deposition method; these composites were applied towards the CO2 reduction reaction (CO2RR). A highlight of this series of experiments was that Au/W2N3 composites were found to be a much more active HER (as opposed to either a CO2RR or a methanol oxidation reaction (MOR)) catalyst.
Ref.: invited contribution, Nano Research, 13(5), 1434-1443 (2020).
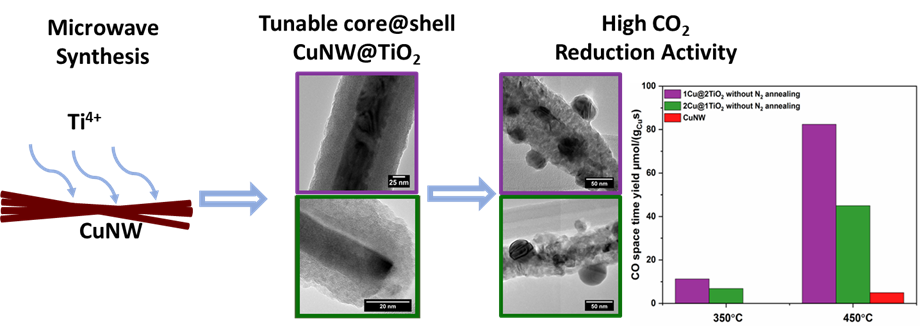
21. The rational synthesis of Cu@TiO2 core@shell nanowire structures was thoroughly explored using a microwave-assisted method through the tuning of experimental parameters such as but not limited to (i) controlled variation in molar ratios, (ii) the effect of discrete Ti precursors, (iii) the method of addition of the precursors themselves, and (iv) time of irradiation. Uniform coatings were obtained using Cu: Ti molar ratios of 1: 2, 1: 1, 2: 1, and 4: 1, respectively. It should be noted that whereas relative molar precursor concentrations primarily determined the magnitude of the resulting shell size, the dependence was non-linear. Moreover, additionally important reaction parameters, such as precursor identity, the means of addition of precursors, and the reaction time, were individually explored with the objective of creating a series of optimized reaction conditions. As compared with Cu nanowires (NWs) alone, it is evident that both of the Cu@TiO2 core-shell NW samples, regardless of pre-treatment conditions, evinced much better catalytic performance, up to as much as 20 times greater activity as compared with standard Cu NWs. These results imply the significance of the Cu/TiO2 interface in terms of promoting CO2 hydrogenation, since TiO2 alone is known to be inert for this reaction. Furthermore, it is additionally notable that the N2 annealing pre-treatment is crucial in terms of preserving the overall Cu@TiO2 core@shell structure. We also systematically analyzed and tracked the structural and chemical evolution of our catalysts before and after the CO2 reduction experiments. Indeed, we discovered that the core@shell wire motif was essentially maintained and conserved after this high-temperature reaction process, thereby accentuating the thermal stability and physical robustness of our as-prepared hierarchical motifs.
Ref.:ACS Applied Materials and Interfaces, 12(29), 32591-32603 (2020).
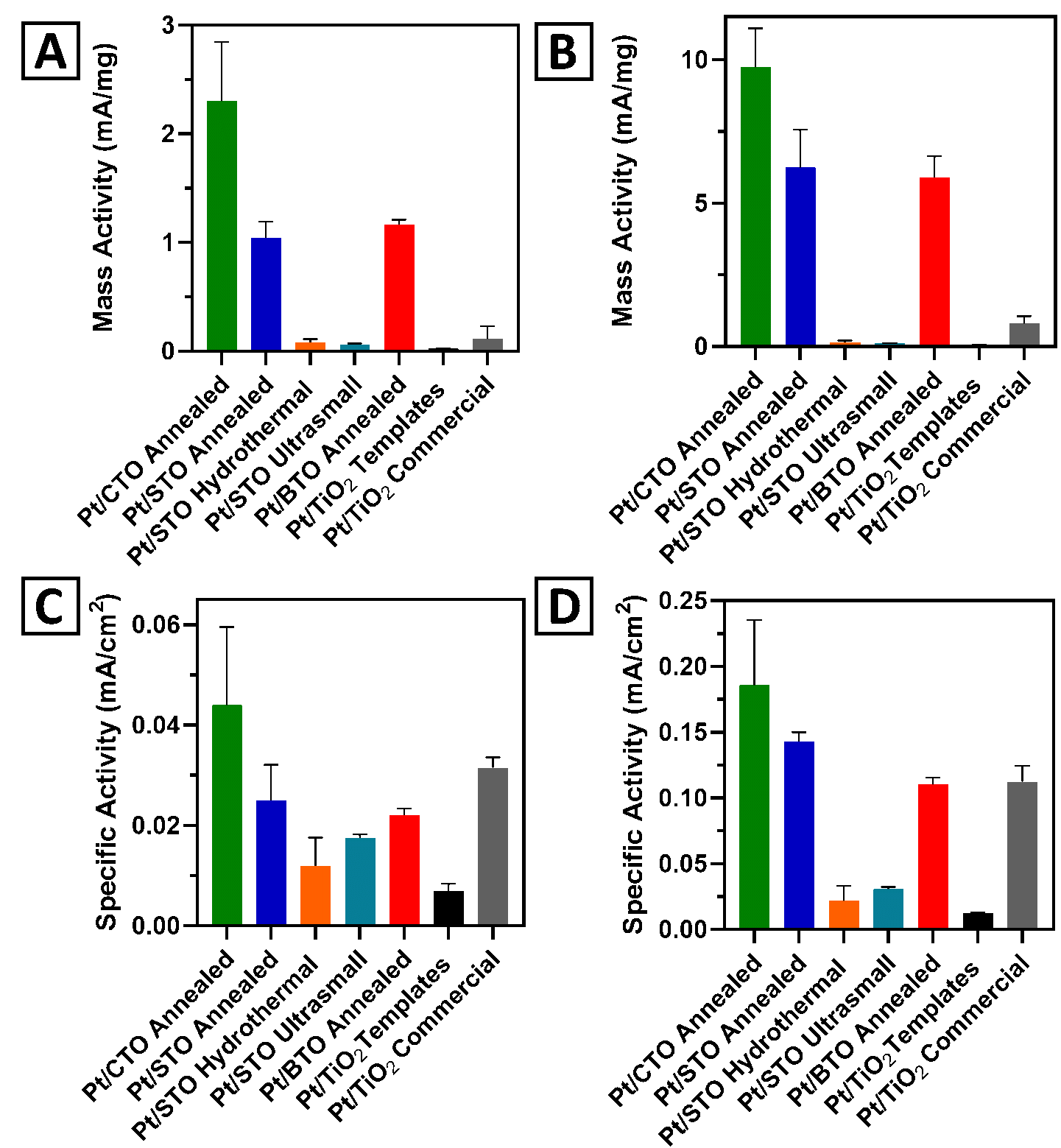
22. We synthesized and subsequently rationalized the formation of a series of 3D hierarchical metal oxide spherical motifs. Specifically, we varied the chemical composition within a family of ATiO3 (wherein “A” = Ca, Sr, and Ba) perovskites, using a two-step, surfactant-free synthesis procedure to generate structures with average diameters of ~3 microns. In terms of demonstrating the practicality of these perovskite materials, we have explored their use as supports for the methanol oxidation reaction (MOR) as a function of their size, morphology, and chemical composition. The MOR activity of our target systems was found to increase with decreasing ionic radius of the “A” site cation, in order of Pt/CaTiO3 (CTO) > Pt/SrTiO3 (STO) > Pt/BaTiO3 (BTO). With respect to morphology, we observed an MOR enhancement of our 3D spherical motifs, as compared with either ultra-small or cubic control samples. Moreover, the Pt/CTO sample yielded not only improved mass and specific activity values but also a greater stability and durability, as compared with both commercial TiO2 nanoparticle standards and precursor TiO2 templates. Ref.: invited contribution, Molecules,26, 909 / 1-23 (2021).
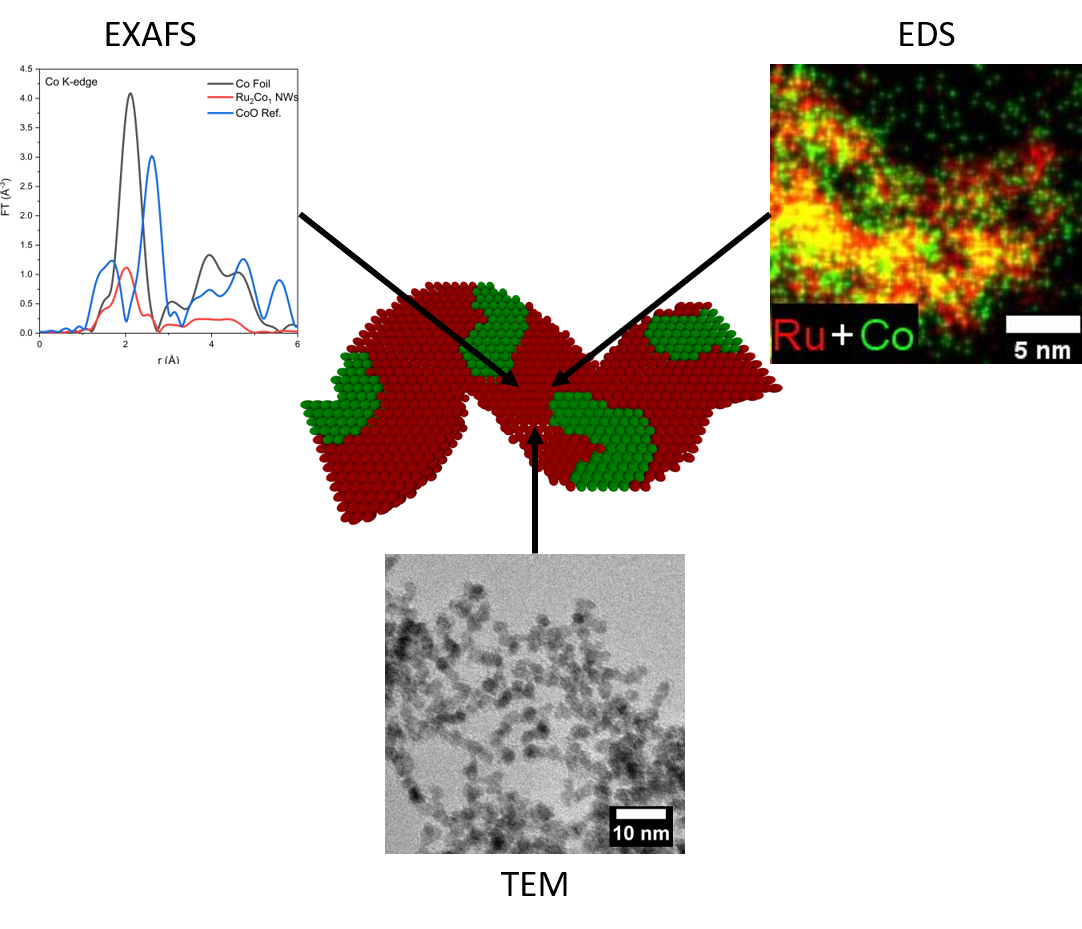
23. A number of complementary, synergistic advances are reported herein. First, we describe the ‘first-time’ synthesis of ultrathin Ru2Co1 nanowires (NWs) possessing average diameters of 2.3 ± 0.5 nm using a modified surfactant-mediated protocol. Second, we utilize a combination of quantitative EDS, EDS mapping (along with accompanying line-scan profiles), and EXAFS spectroscopy results to probe the local atomic structure of not only novel Ru2Co1 NWs but also ‘control’ samples of analogous ultrathin Ru1Pt1, Au1Ag1, Pd1Pt1, and Pd1Pt9 NWs. We demonstrate that ultrathin NWs possess an atomic-level geometry that is fundamentally dependent upon their intrinsic chemical composition. In the case of the PdPt NW series, EDS mapping data are consistent with the formation of a homogeneous alloy, a finding further corroborated by EXAFS analysis. By contrast, EXAFS analysis results for both Ru1Pt1 and Ru2Co1 imply the generation of homophilic structures in which there is a strong tendency for the clustering of ‘like’ atoms; associated EDS results for Ru1Pt1 convey the same conclusion, namely the production of a heterogeneous structure. Conversely, EDS mapping data for Ru2Co1 suggests a uniform distribution of both elements. In the singular case of Au1Ag1, EDS mapping results are suggestive of a homogeneous alloy, whereas EXAFS analysis pointed to Ag segregation at the surface and an Au-rich core, within the context of a core-shell structure. These cumulative outcomes indicate that only a combined consideration of both EDS and EXAFS results can provide for an accurate representation of the local atomic structure of ultrathin NW motifs. Ref.:Chemical Science, 12(20), 7158–7173 (2021).
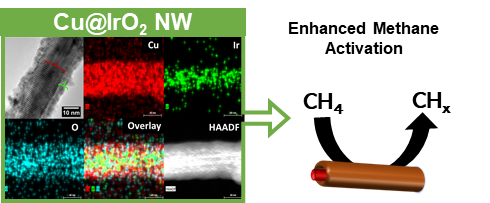
24. A facile microwave-assisted synthesis was developed for the tunable fabrication of a Cu@IrO2 core@shell nanowire motif. Experimental parameters, such as (i) reaction time, (ii) the method of addition of the Ir precursor, (iii) capping agent, (iv) reducing agent, as well as (v) the capping agent-to-reducing agent ratio, were subsequently optimized. The viability of other methods based on previously reported literature, such as refluxing, stirring, and physical sonication, was studied and compared with our optimized microwave-assisted protocol in creating our as-prepared materials. It should be noted that the magnitude of the IrO2 shell could be tailored, based on varying the Cu: Ir ratio coupled with judicious variations in the amounts of capping agent and reducing agent. Structural characterization techniques, such as XRD, XPS, and HRTEM (including HRTEM-EDS), were used to analyze our Cu@IrO2 motifs. Specifically, the shell could be reliably tailored from sizes of 10 nm, 8 nm, 6 nm, and 3.5 nm with corresponding Cu: Ir ratios of 10: 1, 15: 1, 20: 1, and 25: 1, respectively. Moreover, the structural integrity of the motifs was probed and found to have been maintained after not only heat treatment but also the post-methane conversion process, indicative of an intrinsically high stability. Both components within the CuO-IrO2 interface were able to activate methane at temperatures between 400 to 500 K with a reduction of the associated metal cations (Cu2+ à Cu1+; Ir4+ à Ir3+) and the deposition of CHx fragments on the surface, as clearly observed in the ambient-pressure XPS results. Thus, on the basis of their stability and chemical activity, these core-shell materials could be very useful for the catalytic conversion of methane into ‘higher value’ chemicals. Ref.: ACS Applied Nano Materials, 4(10), 11145−11158 (2021)
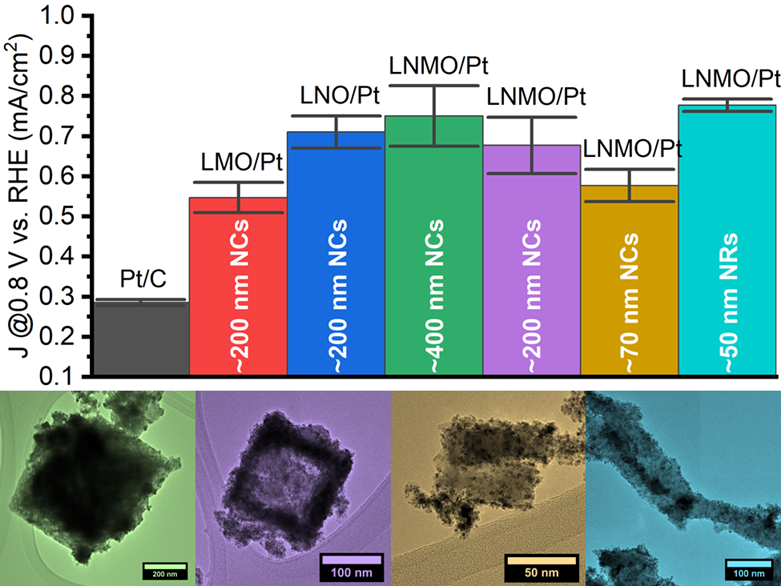
25. We have not only analyzed the performance of perovskite oxides as support media for the methanol oxidation reaction (MOR) but also examined the impact and significance of various reaction parameters on their synthesis. Specifically, we have generated (a) La2NiMnO6, LaMnO3, and LaNiO3 nanocubes with average sizes of ~200 nm, in addition to a series of La2NiMnO6 (b) nanocubes possessing average sizes of ~70 and 400 nm and (c) anisotropic nanorods characterized by average diameters of 40-50 nm. All of these samples, when used as supports for Pt nanoparticles, exhibited activities which were at least twice that measured for Pt/C. We have investigated and correlated the effect of varying perovskite (i) composition, (ii) size, and (iii) morphology upon the measured MOR activity. (i) The Ni-containing perovskites yielded generally higher performance metrics than that of LaMnO3 alone, suggesting that the presence of Ni is favorable for MOR, a finding supported by a shift in the Pt d-band in XPS. (ii) MOR activity is enhanced as the perovskite size increases in magnitude, suggesting that a growth in the perovskite particle size enables favorable, synergistic metal-support interactions. (iii) A comparison of the nanorods and nanocubes of a similar diameter implied that the one-dimensional morphology achieved a greater activity, a finding which can be attributed not only to the anisotropic structure but also to a desirable surface structure. Overall, these data yield key insights into the tuning of metal-support interactions via rational control over the composition, size, and morphology of the underlying catalyst support. Ref.:Catalysis Science and Technology, 12(2), 613–629 (2022).
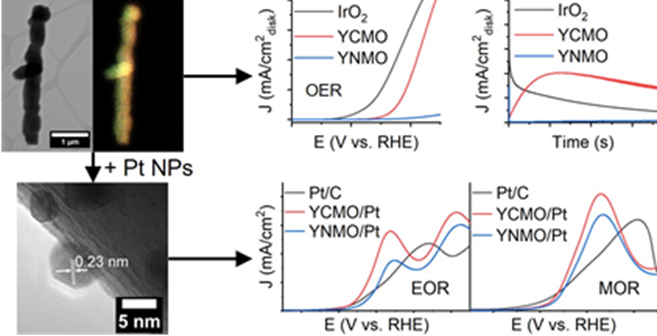
26. Herein, we investigate the effect of chemical composition of double perovskite nanorods on their versatile electrocatalytic activity not only as supports for the oxidation of small organic molecules but also as catalysts for the oxygen evolution reaction. Specifically, Y2CoMnO6 and Y2NiMnO6 nanorods with average diameters of 300 nm were prepared by a two-step hydrothermal method, in which the individual effects of synthetic parameters, such as the pH, annealing temperature, and precursor ratios on both the composition and morphology, were systematically investigated. When used as supports for Pt nanoparticles, Y2CoMnO6/Pt catalysts exhibited an electrocatalytic activity for the methanol oxidation reaction, which is 2.1 and 1.3 times higher than that measured for commercial Pt/C and Y2NiMnO6/Pt. Similarly, the Co-based catalyst support material displayed an ethanol oxidation activity, which is 2.3 times higher than both Pt/C and Y2NiMnO6/Pt. This clear enhancement in the activity for Y2CoMnO6 can largely be attributed to strong metal-support interactions, as evidenced by a downshift in the binding energy of the Pt 4f bands, measured by XPS, which is often correlated not only with a downshift in the d-band center but also to a decreased adsorption of poisoning adsorbates. Moreover, when used as catalysts for the oxygen evolution reaction, Y2CoMnO6 displayed a much greater activity as compared with Y2NiMnO6. This behavior can largely be attributed not only to a preponderance of comparatively more favorable oxidation states and electronic configurations but also to the formation of an active layer on the surface of the Y2CoMnO6 catalyst, which collectively give rise to improved performance metrics and greater stability as compared with both IrO2 and Y2NiMnO6. Overall, these results highlight the importance of both the chemical composition and the electronic structure of double perovskites, especially when utilized in multi-functional roles as either supports or catalysts. Ref.:ACS Applied Materials and Interfaces, 14(27), 30914−30926 (2022).
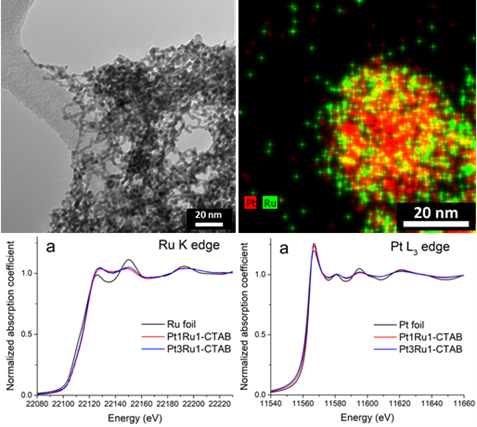
27. In the context of developing novel fuel cell catalysts, we have successfully synthesized in high yields not only ultrathin nanowires with compositions of Pt1Ru1 and Pt3Ru1 but also more complex spoke-like dendritic clusters of Pt1Ru1 and Pt1Ru9 in ambient pressure under relatively straightforward, solution-based reaction conditions, mediated by either CTAB (cetyltrimethylammonium bromide) or oleylamine (OAm), respectively. EXAFS analysis allowed us to determine the homogeneity of as-prepared samples. Based on this analysis, only the Pt3Ru1 sample was found to be relatively homogeneous. All of the other samples yielded results, suggestive of a tendency for the elements to segregate into clusters of ‘like’ atoms. We have also collected complementary HRTEM EDS mapping data, which support the idea of a segregation of elements consistent with the EXAFS results. We attribute the differences in the observed morphologies and elemental distributions within as-prepared samples to the presence of varying surfactants and heating environments, employed in these reactions. Methanol oxidation reaction (MOR) measurements indicated a correlation of specific activity (SA) values not only with intrinsic chemical composition and degree of alloying but also with the reaction process used to generate the nanoscale motifs in the first place. Specifically, the observed performance of samples tested decreased as a function of chemical composition (surfactant used in their synthesis), as follows: Pt3Ru1 (CTAB) > Pt1Ru1 (CTAB) > Pt1Ru1 (OAm) > Pt1Ru9 (OAm). Ref.: invited contribution, Energy Adv.,3(1), 163–176 (2024).
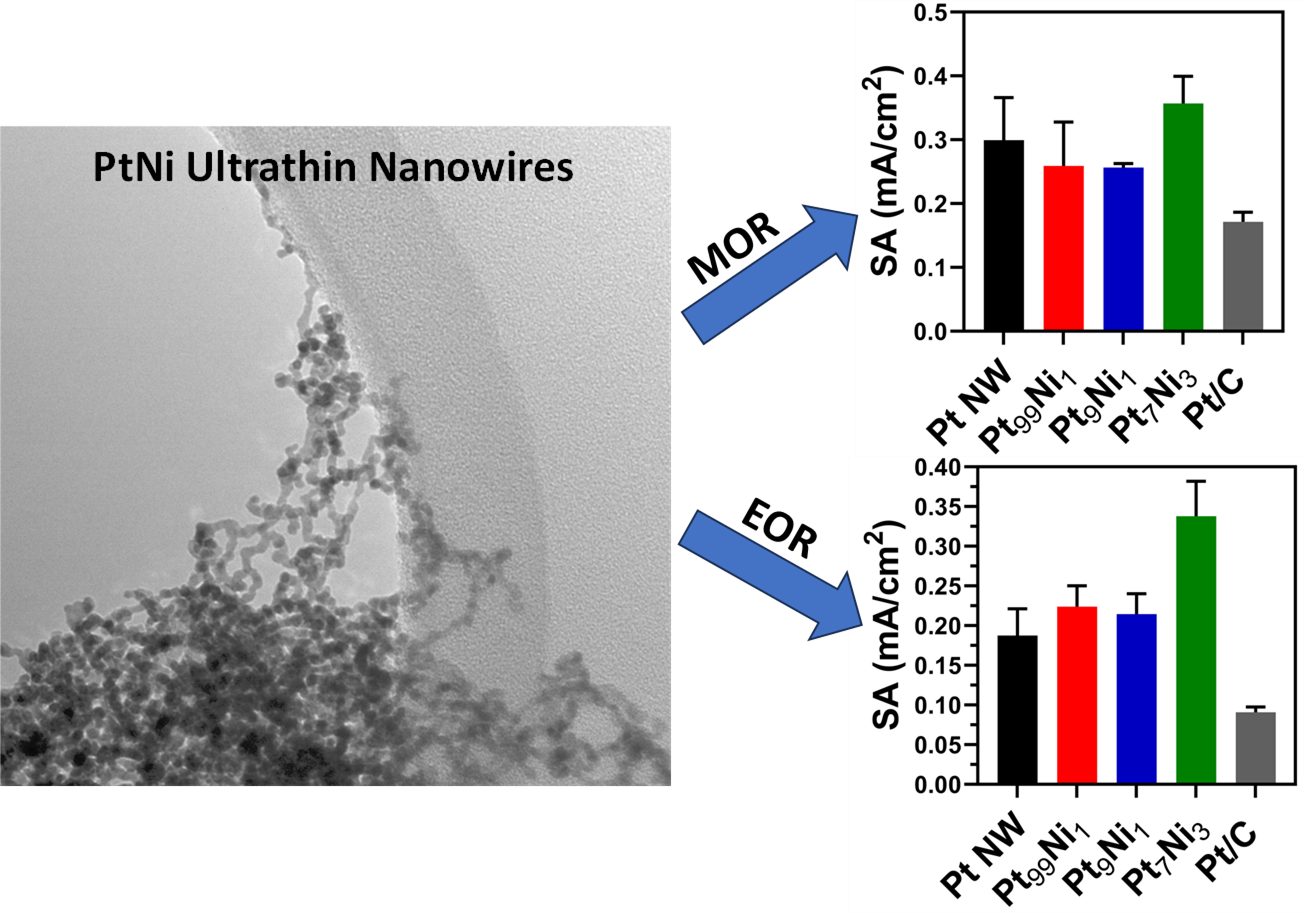
28. We have successfully synthesized ultrathin nanowires of pure Pt, Pt99Ni1, Pt9Ni1, and Pt7Ni3 using a modified room-temperature soft-template method. Analysis of both methanol oxidation reaction (MOR) and ethanol oxidation reaction (EOR) results found that the Pt7Ni3 samples yielded the best performance with specific activities of 0.36 and 0.34 mA/cm2 respectively. Additionally, formic acid oxidation reaction (FAOR) tests noted that both Pt and PtNi nanowires oxidize small organic molecules (SOMs) via an indirect pathway. CO oxidation data suggests little measurable performance without any pre-reduction treatment; however, after annealing in H2, we detected significantly improved CO2 formation for both Pt9Ni1 and Pt7Ni3 motifs. These observations highlight the importance of pre-treating these nanowires under a reducing atmosphere to enhance their performance for CO oxidation. To explain these findings, we collected extended x-ray adsorption fine structure (EXAFS) spectroscopy data, consistent with the presence of partial alloying with a tendency for Pt and Ni to segregate, thereby implying the formation of a Pt rich shell coupled with a Ni rich core. We also observed that the degree of alloying within the nanowires increased after annealing in a reducing atmosphere, a finding deduced through analysis of the coordination numbers and calculations of Cowley’s short range order parameters. Ref.: invited contribution, ChemNanoMat, 10(2), e202300356 / 1-17 (2024).
|